- Therapeutic Cataract & Refractive
- Lens Technology
- Glasses
- Ptosis
- AMD
- COVID-19
- DME
- Ocular Surface Disease
- Optic Relief
- Geographic Atrophy
- Cornea
- Conjunctivitis
- LASIK
- Myopia
- Presbyopia
- Allergy
- Nutrition
- Pediatrics
- Retina
- Cataract
- Contact Lenses
- Lid and Lash
- Dry Eye
- Glaucoma
- Refractive Surgery
- Comanagement
- Blepharitis
- OCT
- Patient Care
- Diabetic Eye Disease
- Technology
Know the ocular effects of tear gas
Water increases the effect of some pepper chemicals


Years ago, I treated a police officer who presented for a dry eye evaluation. While other doctors before me had treated him with tears and punctal plugs, I used steroids and cyclosporine (Restasis, Allergan) to control his ocular inflammation. Following any use of pepper spray, he would see the departmental doctor who would insert plugs to improve his extremely dry eye.
This would inevitably backfire, and within 2 weeks he would return to the office begging me to remove his plugs. Each time I would remove them, treat with a topical steroid taper, and maintain topical cyclosporine. Despite my written instructions on how to better treat his ocular surface following exposure, this cycle went on for years.
Related: 2020 not the year ODs hoped for
As healthcare providers, ODs may be called on to assist those who have been affected by tear gas, pepper spray, and other agents used during periods of unrest. It is not their job to judge but rather to assist these patients by resolving pain and promoting healing. While exposure to these irritants may be common to military medical personnel, civilian practitioners may not be as familiar. This article is not meant to incite political discussion regarding the intended or actual use of these agents. It is to educate readers on the history of and management of exposure to them.
Chemical warfare
To begin, a historical perspective on chemical warfare may be of value. Chinese armies used “stink pots” in 2300 BCE during warfare. They burned red peppers in hot oil to produce offensive smoke to burden their opposition prior to attack. Japanese fighters ground peppers to a fine dust and wrapped the dust in rice paper to throw into the faces of opponents.
Wood saturated with pitch and sulfur was burned to create offensive smoke in the Peloponnesian wars (431 BCE). “Greek fire,” a liquid containing sulfur, lyme, pitch, resin, and petroleum, was pumped into the water in front of ships and burned. Greek fire and hot oil were catapulted over walls in the Middle Ages.1
Related: Handling ocular chemical burns in children and adolescents
Chemical agents in grenades of clay and glass jars were first used in the 13th century. Separate containers would contain two different chemicals, resulting in a chemical reaction when mixed.
Bacteriolgical warfare was first used in 1422 in Italy when cartloads of horse manure were launched over city walls to the detriment of those inside the city of Tarantine.
The Civil War saw the first chemical warfare in America. Wood covered with pitch and sulfur was burned around cities. Greek fire was used on coastal cities.1
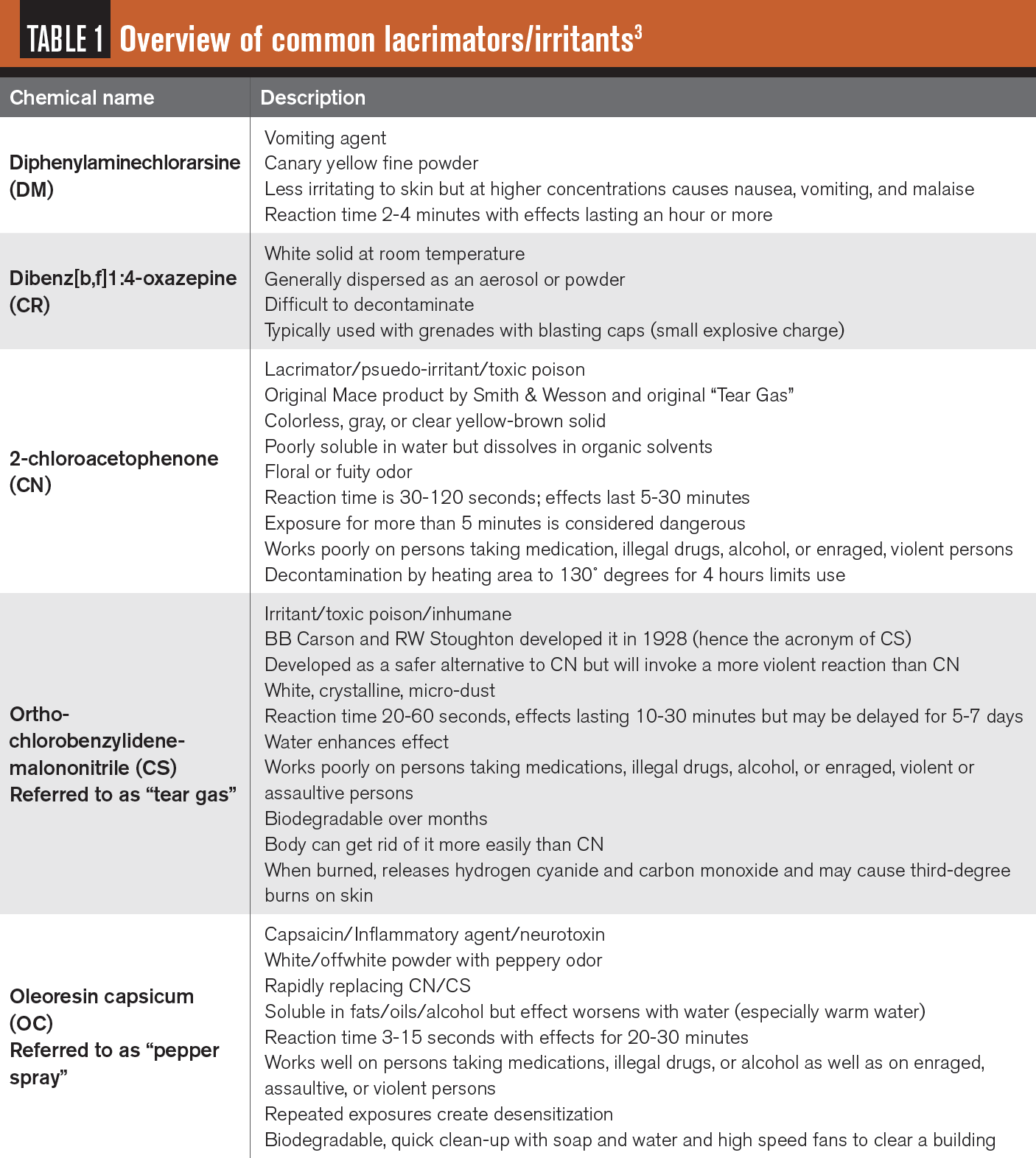
A German chemist developed chloroacetophenone, a chemical lacrimator, in 1869. In 1912, the French used chemical agents in glass containers. A lacrimator called ethyl bromacetate causing severe tearing, blepharospasm, and painful foreign body sensation was used by the French in World War I. In retaliation, Germans used chlorine gas pumped into enemy trenches. British soldiers created gas masks using two surgical masks with sodium carbonate powder between them.1
Related: Why ODs should treat dry eye
During World War I, omega-chloracetophenone (CN) was used to train soldiers using masks. In 1913, Germans used diphenylaminechlorarsine (DM) to make enemies sick, requiring removal of gas masks to strengthen exposure.2 This was later used by British police during civil disorders, resulting in several deaths.1 It was declared a war gas by the United Nations and banned for export from the U.S. in 1958.1
In 1928, scientists developed orthochlorbenzalmalononitrile (CS), which irritated the skin and respiratory system as well as the eyes. Lacrimators and irritants were widely used during World War II. English scientists developed dibenzoxaprine (CR) in 1962, but leaking containers caused third-degree burns on military police so it was discontinued.1
Oleoresin capsicum (OC) was introduced in 1982. This inflammatory agent is produced from cayenne or chili peppers. It causes lacrimation, blepharospasm, and capillary dilation. The mucous membranes are directly affected, causing swelling in the nose throat. Skin inflammation may result. It is the most commonly used agent in policing, riot and crowd control, and self-defense, including defense against attacking animals.3
Related: Lactoferrin levels can diagnose dry eye disease
Effects of these gases
General primary effects include lacrimation, conjunctivitis, sneezing, coughing, reduced vision due to tearing, nasal irritation, mild burning and itching of skin. Blepharospasm, salivation, nasal and throat congestion, dyspnea, nausea and emotional reactions are secondary. Productive cough, contact dermatitis, retching, epistaxis, diarrhea, pulmonary edema, distorted sense of taste may also occur.
High concentrations of CN may cause corneal damage, opacity, and permanent visual impairment. Papulovesicular dermatitis and superficial burns may affect periocular skin. Ingestion of food or water contaminated with CN may result in ulcers, nausea, vomiting and lower GI upset. Exposure to CS may result in abortion or premature labor and delivery in pregnant women. Hydrolysis of CN is slow in aqueous solution, difficult to decompose or decontaminate while CS can be easily inactivated using water with soap.
OC may cause more intense burning to eyes, nose, and respiratory tract than other products, with difficulty breathing due to throat closure, loss of motor skills, and inability to speak.4 OC is also associated with keratitis, conjunctivitis, corneal burns, and damage to nerve fibers.
Related: Homeostasis is the Holy Grail in dry eye disease
Substance P
A peptide neurotransmitter called substance P is generated by nerve endings within the central nervous system (CNS) to transmit pain signals. Upon contact with skin, capsicum causes depletion of substance P, telling the brain the body is suffering life-threatening pain and results in disorientation. When Substance P is applied to small areas, transmission of pain from the area may be reduced, and this treatment may be used to address chronic pain. In the eye, however, this effect may be catastrophic.
Subtance P is also considered a polypeptide neurotoxin because aerosol inhalation causes rapid loss of blood pressure and may result in loss of conscieousness within seconds. Direct skin contact results in a similar but delayed reaction (10 to 15 seconds). Note than those with darker skin will have a significantly delayed reaction of up to 5 minutes. Prior exposure to pepper products will increase response time.
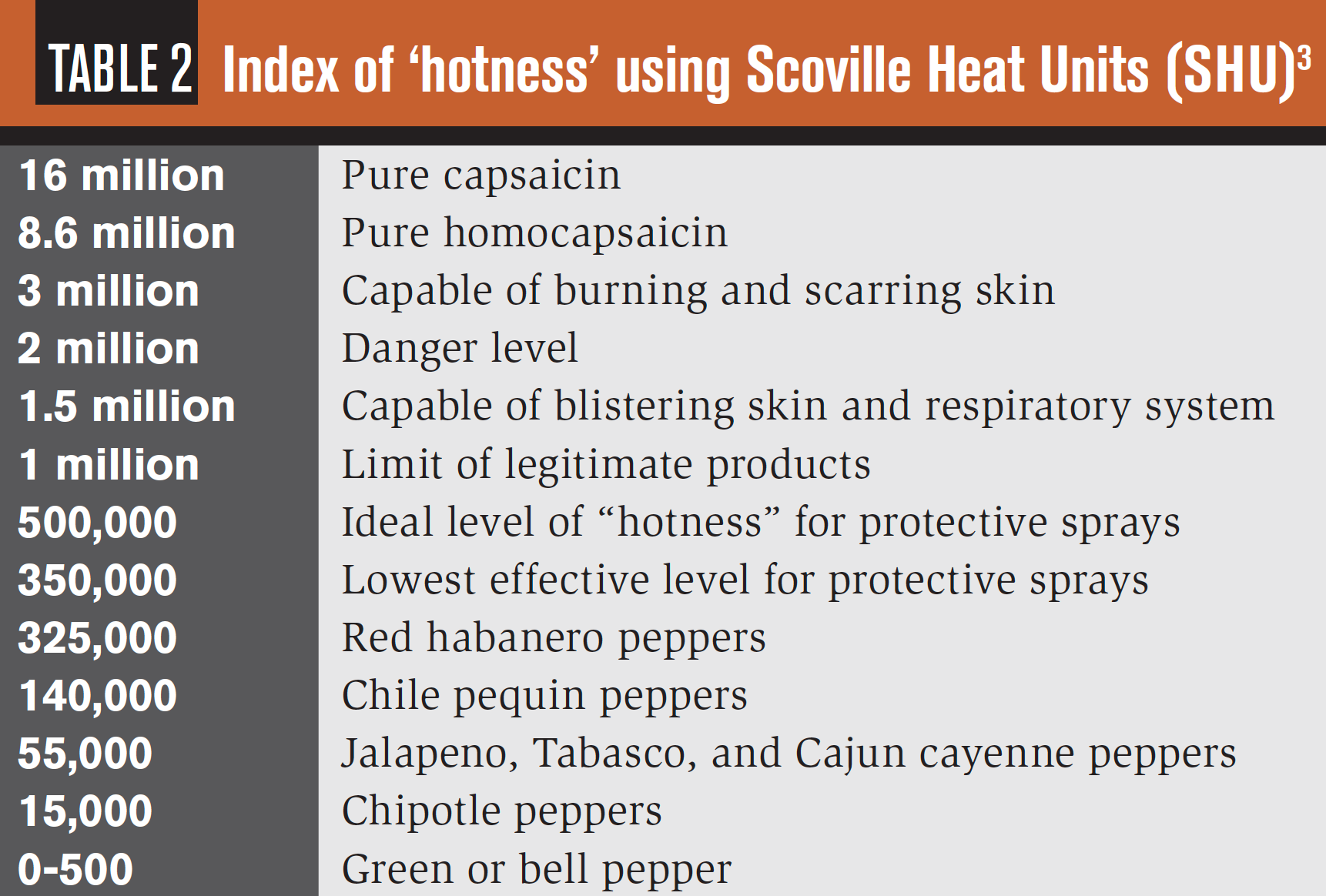
Heat scale
The Scoville Heat Scale was used in the 1920s to assess the “hotness” of certain foods; the foods were mixed with a sugar solution and sampled by five trained testers. This process was abandoned due to subjectivity and replaced by science such as high-pressure liquid chromatography. The unit of measure is the Scoville Heat Unit (SCH). Examples of levels of potency using this measure are noted in Table 2.
Unfortunately, it is common to boost the “hotness” level of OC products without information on boosting released to the public. In addition, different products may be blended to enhance effect. OC/CS combinations are referred to as “Fortified Mace” or “Freeze+P.”
Molecular targets
The molecular target of capsaicin TRPV1 is a transient receptor potential (TRP) ion channel expressed in nociceptors.5 These receptors are part of the pain-sensing sensory nerves of the trigeminal, vagal, and dorsal root ganglia (DRG). Nociceptors are located in the skin, cornea, and conjunctiva, as well as in mucous membranes of the respiratory system. TRPV1 promotes neuronal depolarization when activated, acting as a warning sensor for imminent tissue damage.5 TRPV1 is sensitized through signaling from a range of G protein–coupled receptors including bradykinin, prostaglandin, nerve growth factor, cytokine and chemokine receptors.
The tear gas agents CS, CN, and CR have been linked to a single target, TRPA1, despite being structurally dissimilar. TRPA1 is also a TRP ion channel expressed in nociceptors. Pain neurobiological research has demonstrated that TRPA1 is also the target of mustard oil, wasabi, and horseradish. This will not come as a surprise to anyone eating these foods by mistake.5
Ocular and nasal pain are sensed almost immediately after exposure to CN, CR, and CS as well as OC due to activation of TRPV1 and TRPA1 receptors in trigeminal nerve endings in the cornea and nasal passages. Chronic TRPA1 activity creates nerve calcium overload and excitotoxicity. This results in chronic pain signaling and peripheral neurodegeneration, a loss of sensation, or altered sensations.5 When ligands of TRPV1 and TRPA1 are applied topically, extensive desensitization and remodeling of cutaneous nerve endings may occur.
Corneal TRPV1 and TRPA1 activation provides immune responses to noxious environmental stresses such as temperature fluctuations, rises in ambient osmolarity, mechanical stretch, decline in pH, tissue injury, and exposure to irritant ligands.6 Expression of TRPV1 is demonstrated in the corneal epithelial cell membrane in addition to corneal nerve fibers.6 This is particular disconcerting for patients with diabetes and pre-existing neurotrophic corneal disease.
Ocular effects
As previously stated, the most common effects of these agents include lacrimation, blepharospasm, conjunctivitis, keratitis, periorbital erythema, and edema.
Photophobia may occur. Because CR/CN/CS are solids, dust may be captured beneath the eyelid or embedded in the cornea or conjunctival tissue and require removal.1 OC solvents may cause corneal abrasions.
Periocular skin erythema and pain may be significant, particularly when the skin is raw or in high temperatures, humidity, or agent concentration. Erythema may occur hours after CS exposure, followed by vesication. This appears similar to a severe sunburn.1 Oral steroids may be required for severe dermatitis.8
A study of 81 emergency department patients presenting for treatment following exposure to OC found ocular pain and redness were the most common presenting signs. Corneal abrasions and respiratory symptoms occurred with essentially identical frequency (7 and 6 patients, respectively).9
Another study of ocular effects including contact lens wear was conducted with 22 police officers. Snellen acuity and anterior segment appearance were evaluated. Four brands of contact lenses were sprayed, cleaned twice using alcohol-based cleaner, and evaluated using gas chromatography. Acute ocular effects lasted 5 to 10 minutes with complete recovery in 60 minutes. All suffered conjunctivitis. Keratoepithelial defects (KEDs) were reported upon instillation of fluorescein stain. KEDs healed within 24 hours without treatment. Residue was noted on contact lenses despite cleaning. Recommendations included palliative treatment with water and immediate removal and disposal of contact lenses if exposed to OC.10
Some evidence indicates that OC temporarily inhibits the blink reflex and limits responsiveness to mechanical and chemical stimuli to the eye.11 Recurrent corneal erosions have also been reported.12
More severe injuries to gas exposures have been reported including corneal stromal edema, conjunctival tearing, deep cornea deep vascularization vitreous hemorrhage, traumatic optic neuropathy, symblepharon, pseudopterygium, infective keratitis, trophic keratopathy, glaucoma, and cataracts.13

Decontamination
Following are recommendations to decontaminate eyes and skin.
Eyes: Thoroughly flush with water, saline, or similar substance. Note that water increases the effect of OC, so copious volumes should be used. Ensure no particles remain in ocular tissue. A study comparing the use of water versus baby shampoo with water found the use of baby shampoo was unhelpful following exposure to CS and OC.14
Skin (CS, CN, CR, and DM): Flush with copious amounts of water, soap and water, or a mildly alkaline solution (sodium bicarbonate or sodium carbonate). Decontamination is not needed if the wind is brisk and particles may be shaken off clothing and hair.7
Skin (OC): The pain from OC will increase with water, especially warm water. It is best to decontaminate with baby shampoo, milk (which contains casein), alcohol, or vegetable oil. Without decontamination, pain will subside over time.1
When it happens
Educate patients regarding delayed symptoms and signs of increased ocular surface disease. Patients should be instructed to contact the OD should symptoms progress rather than resolve. Autoinflammatory treatments such as hypochlorous acid, topical steroids, and NSAIDs may be beneficial. For those with repeated exposure, periodic evaluations to assess corneal health may be advised.
More by Dr. Schroeder Swartz: How to create an ocular surface disease treatment protocol
References:
1. U.S. Army Medical Research Institute of Chemical Defense (2015-03-21) FE. RIOT-Control Agents. In: Medical Management of Chemical Casualties Handbook. Fort Sam Houston, Texas: Office of The Surgeon General, Borden Institute; 2014:103-114.
2. Patton J. Gas in the Great War. University of Kansas Medical Center. Available at: http://www.kumc.edu/wwi/medicine/gas-in-the-great-war.html. Accessed 6/30/20.
3. Faulkner JR. National Institute of Corrections Community Corrections Division Chemical Agent Instructor’s Manual. Washington, DC: Department of Justice National Institute of Corrections; 1990. Available at: https://www.ncjrs.gov/pdffiles1/Digitization/146592NCJRS.pdf. Accessed 6/17/20.
4. Atkinson JM. Advanced Chemical Weapons Course: Advanced Chemical Weapons. Gloucester, MA: Granite Island Group; 1997.
5. Rothenberg C, Achanta S, Svendsen ER, Jordt SE. Tear gas: an epidemiological and mechanistic reassessment. Ann N Y Acad Sci. 2016 Aug;1378(1):96-107.
6. Okada Y, Reinach PS, Shirai K, et al. Transient Receptor Potential Channels and Corneal Stromal Inflammation. Cornea. 2015 Nov;34 Suppl 11:S136-141.
7. Eguchi H, Hiura A, Nakagawa H, et al. Corneal Nerve Fiber Structure, Its Role in Corneal Function, and Its Changes in Corneal Diseases. Biomed Res Int. 2017;2017:3242649.
8. Schep LJ, Slaughter RJ, McBride DI. Riot control agents: the tear gases CN, CS and OC-a medical review. J R Army Med Corps. 2015 Jun;161(2):94-99.
9. Watson WA, Stremel KR, Westdorp EJ. Oleoresin capsicum (Cap-Stun) toxicity from aerosol exposure. Ann Pharmacother. 1996 Jul-Aug;30(7-8):733-735.
10. Lee RJ, Yolton RL, Yolton DP, Schnider C, Janin ML. Personal defense sprays: effects and management of exposure. J Am Optom Assoc. 1996 Sep;67(9):548-560.
11. Olajos EJ, Salem H. Riot control agents: pharmacology, toxicology, biochemistry and chemistry. J Appl Toxicol. 2001 Sep-Oct;21(5):355-391.
12. Holopainen JM, Moilanen JA, Hack T, Tervo TM. Toxic carriers in pepper sprays may cause corneal erosion. Toxicol Appl Pharmacol. 2003 Feb 1;186(3):155-162.
13. Gray PJ, Murray V. Treating CS gas injuries to the eye. Exposure at close range is particularly dangerous. BMJ. 1995 Sep 30;311(7009):871.
14. Stopyra JP, Winslow JE 3rd, Johnson JC 3rd, et al. Baby Shampoo to Relieve the Discomfort of Tear Gas and Pepper Spray Exposure: A Randomized Controlled Trial. West J Emerg Med. 2018 Mar;19(2):294-300.

