Positive phase 3 results from NOV03 drug trial for MGD
Both co-primary end points were achieved
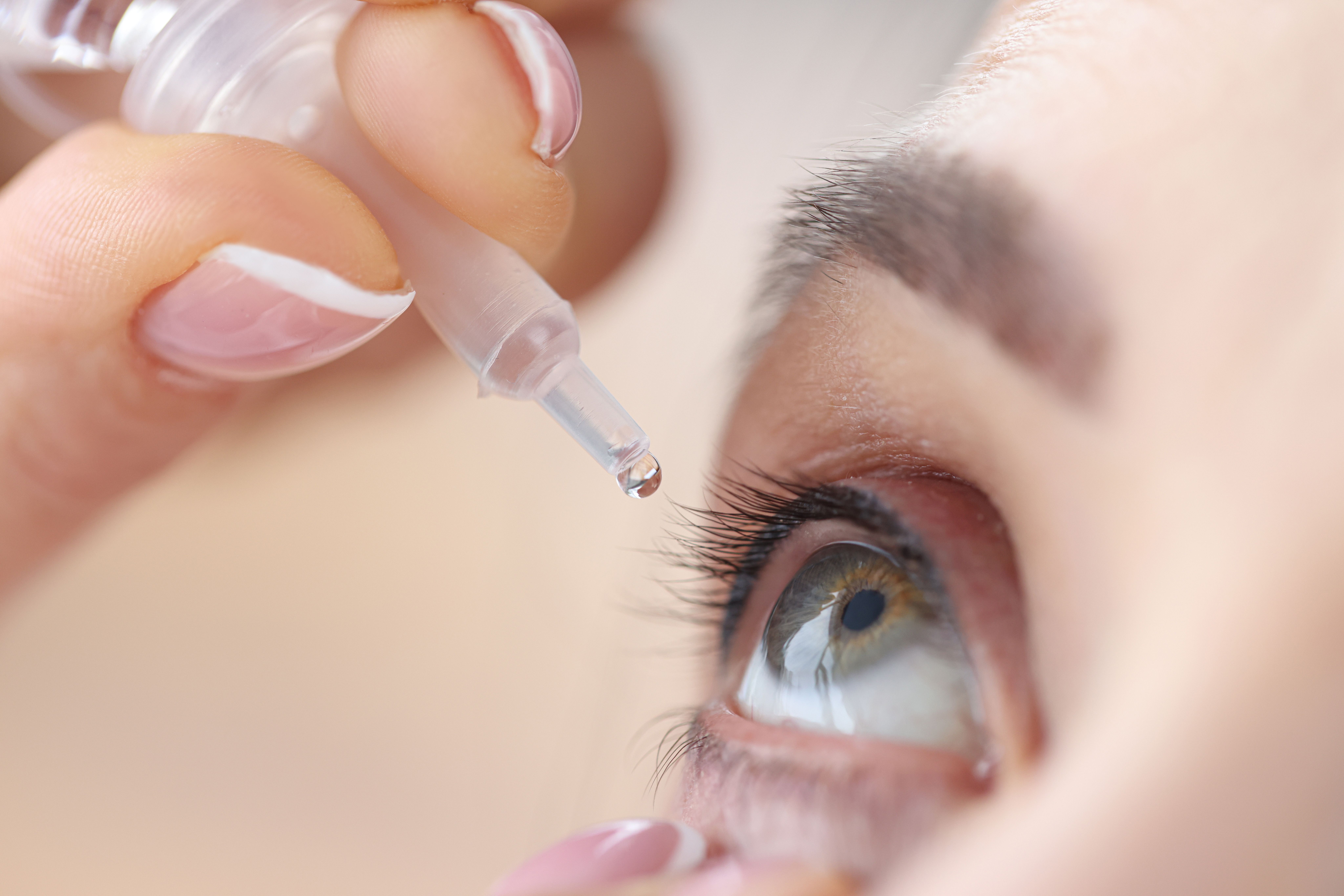
Novaliq and Bausch + Lomb announced topline data from the first phase 3 trial of NOV03, a novel eye drop indicated for the treatment of dry eye disease.
Co-primary endpoints
The GOBI trial met its co-primary end points in the treatment of dry eye disease and assessed the effects of Total Corneal Fluoresceolin Staining (tCFS) on eye damage. The co-primary end points included:
· tCFS changes from baseline. This measure reached statistical significance at day 15. It was also observed that continued results were achieved through day 57.1
· Improvement in the dryness score. Statistically significant results were observed by day 15. Statistical significance sustained through day 57, with continued results through the primary endpoint.1
Secondary end point
The GOBI trial also met its secondary end point, the reduction of DED symptoms in patients with meibomian gland disfunction (MGD).2
“The rapid onset of action and statistical significance demonstrated in this trial is impressive,” says Joseph C. Papa, chairman and CEO, Bausch Health, in a statement. “It also gets us a step closer to bringing forward this potential first-in-class treatment option, which could be a promising development for millions of patients.”
The company’s phase 3 program includes 2 studies, the multicenter MOJAVE trial and the single-arm KALAHARI study. The goal of these trials is to establish safety and efficacy data for NOV03.2 With positive topline results, Novaliq would be able to file NOV03 with the Food and Drug Administration (FDA) in 2022.
Study information
The study focused on 597 participants who were randomized to receive NOV03 or placebo and included 4 secondary end points, which indicated statistical superiority over placebo in terms of change from baseline in 3 different measures of tCFS.2 These included change from baseline in the dryness score, visual analog scale (VAS) burn/stinging, and central corneal fluorescence staining.
No instillation site reactions were reported in the treatment group. No treatment emergent adverse event occurred in the control group.
References
1. Tauber J, Wirta DL, Sall K, Majmudar PA, et al. A randomized clinical study (SEECASE) to assess efficacy, safety, and tolerability of NOV03 for treatment of dry eye disease, Cornea. December 22, 2020 - Volume Publish Ahead of Print - Issue - doi:10.1097/ICO.0000000000002622
2. Bausch + Lomb announces statistically significant topline results from the first phase 3 trial of NOV03 (perfluorohexyloctane) in dry eye disease associated with meibomian gland dysfunction. News release. Bausch + Lomb. April 13, 2021. Accessed May 17, 2021.