Virtual reality, tablet devices capture visual fields in unconventional ways

Visual field testing is an integral component of contemporary clinical practice because it measures outcomes that correlate directly with a patient's quality of life.1-4
New devices and technology provide visual field assessments in unconventional ways-away from clinical settings and/or with indirect supervision of a trained clinician.
Among these are tablet-based devices and head-mounted displays. Conducting visual field testing on a tablet has obvious limitations. If the test is unsupervised, then lighting conditions, viewing distance, and screen brightness are difficult to control.5
Related: Using virtual reality in your practice
Head-mounted and virtual reality (VR) systems are not burdened with these limitations.
VR headsets are inexpensive, readily available, lightweight, mobile, easy to transport to use for screening outside of the clinic, and they can be used with a variety of input devices.
Head, hand, and eye tracking can be used as inputs, as can both traditional (Xbox, Bluetooth Android) and positionally tracked (Oculus Touch, HTC Vive, Google Daydream View) game controllers.
Because most headsets are worn by the patient, patients do not have to keep their heads stationary in a chin/forehead rest. Headsets are able to be used while sitting, standing, or lying down. Alternatively, headsets can be mounted on a stationary stand while using hand or eye tracking in place of a response button.
Why innovation is needed
The most common form of visual field testing in practice is static automated perimetry (SAP). It is used primarily as a functional assessment to assist in the diagnosis and management of diseases of the eye, optic nerve, and visual pathway.
SAP, however, is not typically used routinely. Here’s why.
• It is time consuming (it takes four to seven minutes per eye)
• Patients new to the device often fail even if they are healthy (many false positives)6-10
• Prevalence of true positives is low (three to five percent of the general population has a visual field loss; that number climbs to 13 percent for patients over age 65)11
• There is cost associated with performing the test, and there is no reimbursement in the absence of a corresponding diagnosis code
As a consequence, SAP is typically used to refine a screening analysis, to assess the presence of functional loss when it is suspected (for example, in the setting of high intraocular pressure [IOP] or suspicious appearance of the optic nerve/retinal nerve fiber layer) or to monitor the stability or progression of disease.
Related: How to determine cause of visual field loss
Unfortunately, the value of SAP is limited in many patients by their inability to give accurate test results. With annual visual field testing in glaucoma, for example, it can take five years or more to detect even rapid visual field progression (2 decibels loss [dB]/year).12
It is possible to improve the ability to reliably estimate progression rates within a shorter period if more visual fields are used. However, despite the potential benefits of increased testing frequency, typical glaucoma patients receive only two to three visual fields in the first two years after diagnosis. In fact, some patients take in excess of 10 years to obtain the six visual fields recommended by some clinical guidelines.13-15
It would therefore be useful to consider alternative ways to acquire visual field data.
Several head-mounted visual field devices available in various stages of development.16-20 The “imo” (CREWT Medical Systems) and Vivid Vision Perimetry (Vivid Vision Inc.) are expected to be on the market soon, and Virtual Field was launched in fall 2018.

“imo”
This head-mounted perimeter “imo” (Figure 1) consists of a main perimeter unit, a user control tablet, and a patient response button.20 A computer unit and a lithium-ion battery are built in the perimeter unit (W22 cm × D38 cm × H24 cm, 1.8 kg).
During testing, the examiner operates the control tablet connected to the perimeter unit by Wi-Fi. Patient responses are obtained using the response button connected by Bluetooth. A stationary stand is available if the patient prefers not to wear the device.
During the exam, a test target is displayed using two sets of full high-definition (HD) transmissive liquid crystal displays and high-intensity light emitting diode (LED) backlights separately for the right and left eyes. The unit has built-in eye tracking and many adjustments, including for interpupillary distance.
Spherical lens correction between -9.00 D to +3.00 D can be accounted for with internal focusable lenses. Astigmatic correction requires an additional removable magnetic cylindrical lens system.
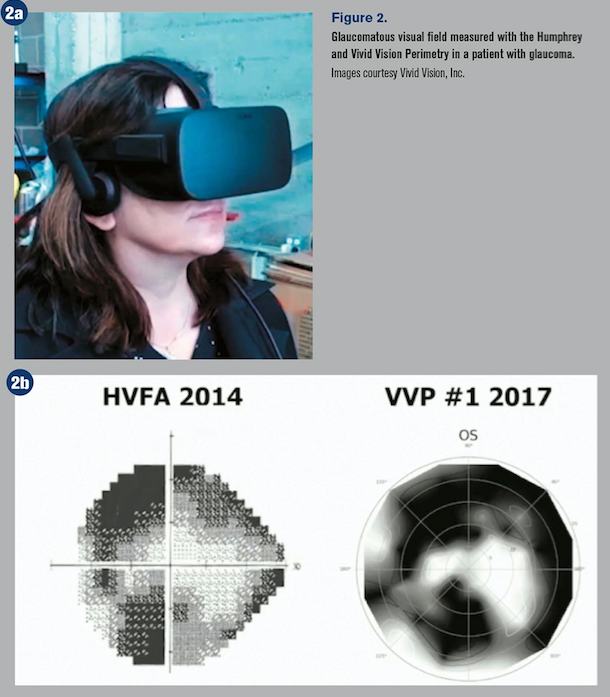
Vivid Vision
The Vivid Vision perimeter (VVP) is a software product that runs on consumer-grade VR headsets, such as Oculus Rift and mobile headsets that do not require a separate computer.
Most VR headsets weigh about 500 g, which is too heavy for frail adults unless testing is performed supine.
VVP was originally developed from the Damato test21 in which patients are encouraged to move their heads and eyes during the test. The fixation marker changes location from trial to trial, and rather than monitoring eye position with a camera, the test exploits the fact that patients fixate accurately when performing high-precision tasks.
Patients respond to targets by moving their heads or a hand-held pointer in the direction of seen targets, which allows false positive responses to be properly classified on a trial-by-trial basis.
Related: Optical coherence tomography and visual field testing both essential
VR headsets used with the VVP typically have fixed focus optics-often set at about 2 m-but commercially available lens holders can be used when needed.
The perimeter has a programmable interface and several pre-programmed tests, one of which is similar to a traditional 24-2 test. Two other tests use dark-on-light targets with locations in a radial pattern, which are intended for vision screening, and measuring fields with known defects, respectively.
As with “imo,” stimuli for the left and right eyes are intermixed into a single test lasting six to eight minutes. Maximum luminance in Oculus Rift is 250 apostib (80 cd/m2), which is lower than traditional SAP tests can provide.
Virtual Field
Virtual Field, Inc. launched its VR platform at the American Academy of Optometry meeting in November.22
The system includes a VR headset, computer, and clicker. Available tests are traditional 24-2 and suprathreshold VF tests. The company plans to release as platform updates 10-2, 30-2, kinetc, and frequencing doubling tests.
According to the company, the device tests both eyes simultaneously without requiring an eye patch. It also features fixation and sleep monitoring to reduce invalid test results.
The future of field testing
Static automated visual field tests are time consuming and often disliked by patients. The devices require dedicated office space and technicians to administer and observe the test, and testing must occur one eye at a time.
Head-mounted VR displays make it possible to give visual field tests that are bilateral, mobile, inexpensive, and could be conducted more frequently and inexpensively-even at home.
References:
1. Alqudah A, Mansberger SL, Gardiner SK, Demirel S. Vision-related quality of life in glaucoma suspect or early glaucoma patients. J Glaucoma. 2016 Aug;25(8):629-33.
2. Nelson P, Aspinall P, Papasouliotis O, Worton B, O'Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma. 2003 Apr;12(2):139-50.
3. McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R; Los Angeles Latino Eye Study Group. Impact of visual field loss on health-related quality of life in glaucoma: The Los Angeles Latino Eye Study. Ophthalmology. 2008 Jun;115(6):941-948.e1.
4. Gutierrez P, Wilson M, Johnson C, Gordon M, Cioffi GA, Ritch R, Sherwood M, Meng K, Mangione CM. Influence of glaucomatous visual field loss Ãon health-related quality of life. Arch Ophthalmol. 1997 Jun;115(6):777-84.
5. Turpin A, Lawson DJ, McKendrick AM. PsyPad: a platform for visual psychophysics on the iPad. J Vis. 2014 Mar 11;14(3):16.
6. Heijl A, Lindgren G, Olsson J. The effect of perimetric experience in normal subjects. Arch Ophthalmol. 1989 Jan;107(1):81-6.
7. Katz J, Sommer A. Screening for glaucomatous visual field loss. The effect of patient reliability. Ophthalmology. 1990 Aug;97(8):1032-7.
8. Schimiti RB, Avelino RR, Kara-José N, Costa VP Full-threshold versus Swedish Interactive Threshold Algorithm (SITA) in normal individuals undergoing automated perimetry for the first time. Ophthalmology. 2002 Nov;109(11):2084-92; discussion 2092.
9. Castro DP, Kawase J, Melo LA Jr. Learning effect of standard automated perimetry in healthy individuals. Arq Bras Oftalmol. 2008 Jul-Aug;71(4):523-8.
10. Johnson CA, Keltner JL, Cello KE, Edwards M, Kass MA, Gordon MO, Budenz DL, Gaasterland DE, Werner E; Ocular Hypertension Study Group. Baseline visual field characteristics in the ocular hypertension treatment study. Ophthalmology. 2002 Mar;109(3):432-7.
11. Ramrattan RS, Wolfs RC, Panda-Jonas S, Jonas JB, Bakker D, Pols HA, Hofman A, de Jong PT. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: the Rotterdam Study. Arch Ophthalmol. 2001 Dec;119(12):1788-94.
12. Jansonius NM. On the accuracy of measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2010 Oct;94(10):1404-5.
13. Fung SS, Lerner C, Russell RA, Malik R, Crabb DP. Are practical recommendations practised? A national multi-centre cross-sectional study on frequency of visual field testing in glaucoma. Br J Ophthalmol. 2013 Jul;97(7):843-7.
14. National Health and Medical Research Council. NHMRC Guidelines for the Screening, Prognosis, Diagnosis, Management and Prevention of Glaucoma 2010. Canberra, ACT: National Health and Medical Research Council; 2010. Available at: http://www.icoph.org/dynamic/attachments/resources/australia_and_new_zealand_glaucoma_guidelines.pdf. Accessed 11/19/18.
15. European Glaucoma Society. Terminology and Guidelines for Glaucoma. 4th ed. Available at: https://bjo.bmj.com/content/bjophthalmol/101/4/1.full.pdf. Accessed 11/19/18.
16. Hollander DA, Volpe NJ, Moster ML, Liu GT, Balcer LJ, Judy KD, Galetta SL. Use of a portable head mounted perimetry system to assess bedside visual fields. Br J Ophthalmol. 2000 Oct;84(10):1185-90.
17. Nagata S, Kani K. A new perimetry based on eye movement. In: A. H, editor. Perimetry Update 1988/1989. Amsterdam Berkeley, Milano: Kugler & Ghedini; 1997.
18. Wroblewski D, Francis BA, Sadun A, Vakili G, Chopra V. Testing of visual field with virtual reality goggles in manual and visual grasp modes. Biomed Res Int. 2014;2014:206082.
19. Tsapakis S, Papaconstantinou D, Diagourtas A, Droutsas K, Andreanos K, Moschos MM, Brouzas D. Visual field examination method using virtual reality glasses compared with the Humphrey perimeter. Clin Ophthalmol. 2017 Aug 7;11:1431-1443.
20. Matsumoto C, Yamao S, Nomoto H, Takada S, Okuyama S, Kimura S, Yamanaka K, Aihara M, Shimomura Y. Visual field testing with head-mounted perimeter ‘imo’. PLoS One. 2016 Aug 26;11(8):e0161974.
21. Damato BE. Oculokinetic perimetry: a simple visual field test for use in the community. Br J Ophthalmol. 1985 Dec;69(12):927-31.
22. Virtual Field website. Available at: https://www.virtualfield.io. Accessed 11/19/18.
