Drug therapy improves prognosis of retinal vein blockage
Advances in pharmacotherapy, including intravitreal administration of corticosteroids and inhibitors of vascular endothelial growth factor, have improved management of retinal vein occlusions.
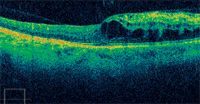
Optometrists can play an important role in optimizing patients' visual and general health prognosis by providing appropriate referral to a retinal specialist and the patient's primary-care provider for evaluation of possible underlying systemic disease, said Dr. Haynie, executive clinical director, Retina and Macula Specialists, Tacoma, WA.
Optometrists are often the first eye-care professionals to see patients with branch and central retinal vein occlusions (BRVO and CRVO, respectively). These patients develop a sudden, painless onset of vision loss. Either diagnosis carries a risk for permanent vision loss, but timely treatment and continued monitoring may reduce that risk, he said.
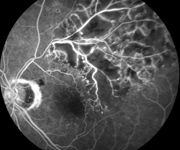
Accumulating experience indicates, however, that initiation of corticosteroid treatment for macular edema, or alternatives when corticosteroids are contraindicated, may hasten and improve visual recovery after BRVO.
"If the vision loss is related to capillary nonperfusion, it is likely to be permanent," Dr. Haynie said. "However, patients may be [treated with] brimonidine tartrate (Alphagan/Alphagan P, Allergan) for possible ischemic neuroprotection. Moreover, chronic macular edema is also a cause of permanent vision loss after BRVO, and the sooner resolution is achieved, the greater the likelihood of improving the final visual outcome.

Aggressive therapy
How aggressive one becomes with the pharmacologic treatment may be based on the severity of the condition, balancing the risks of treatment against its benefits. The least invasive treatment is to start topical corticosteroids or nonsteroidal anti-inflammatory drugs q.i.d. for 6 to 8 weeks. More aggressive treatment can also be considered, including a sub-Tenon's or intravitreal injection of triamcinolone acetonide or an anti-VEGF compound, such as bevacizumab or ranibizumab (Avastin or Lucentis, Genentech). Use of bevacizumab for this purpose is considered off-label.
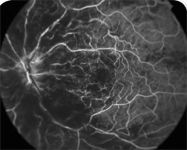
Intravitreal triamcinolone has been reported to resolve macular edema in more than 80% of patients with BRVO. It can work rapidly, within a week in some cases, but it may take 6 to 8 weeks. In addition to a higher risk of elevated IOP and glaucoma, an intravitreal injection is associated with a higher incidence of cataracts, ptosis, and endophthalmitis.
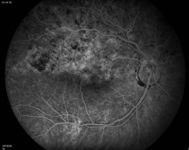
"This low dose of acetazolamide is generally very safe. However, acetazolamide should not be used in patients with renal impairment, a known sulfa allergy, or a diabetic patient without first consulting the primary care provider," Dr. Haynie said.

Optometrists should also remember that 80% to 90% of patients with a BRVO have underlying systemic hypertension.
"Identifying hypertension and providing an appropriate referral can make a huge life difference in these patients," Dr. Haynie said.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.