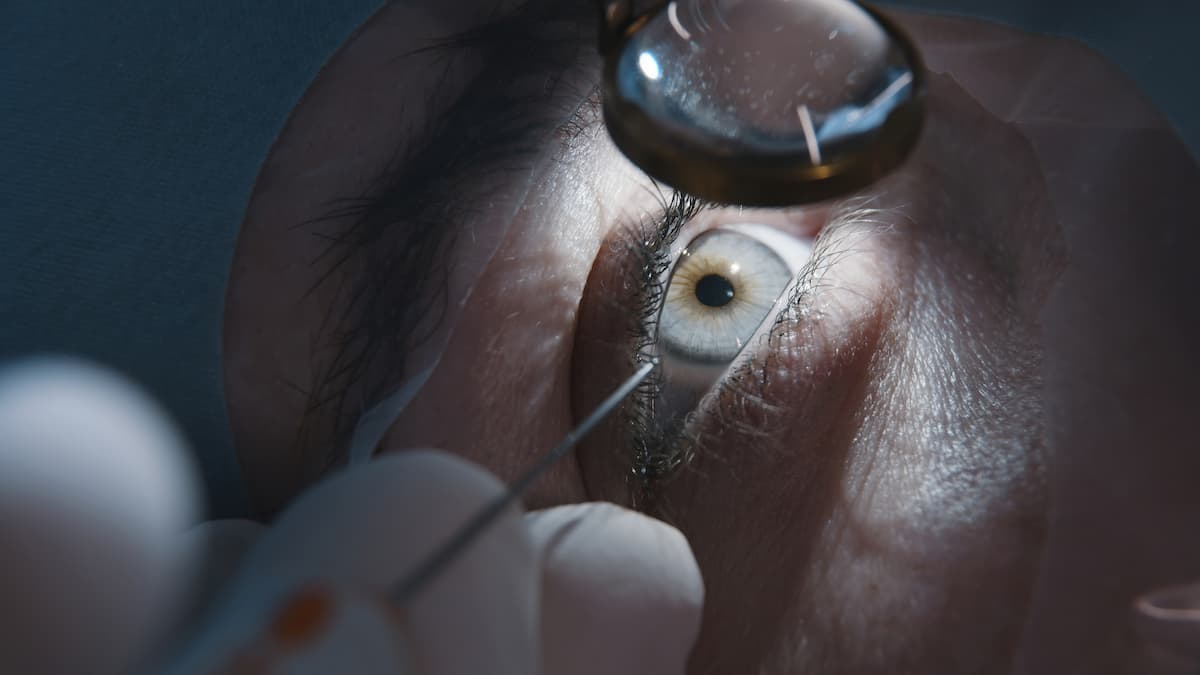
Presbyopia
Latest News
Latest Videos

Podcasts
More News

Commercial product shipments will be initiated for patients in October through LENZ’s ePharmacy partner, and the product is anticipated to be available in commercial locations such as retail pharmacies by the middle of the fourth quarter of this year.

The lens will be available to order on September 17 in the US.

The trial will evaluate the solution in treating significant, chronic night driving impairment in keratorefractive patients with reduced mesopic vision.

The latest in drug and technology developments and updates from July 2025.

Vizz (LENZ Therapeutics) is the first and only aceclidine-based eye drop for presbyopia and is also the first daily solution to correct vision for up to 10 hours.

Beginning in July 21, the Varilux Physio extensee and Varilux Physio extensee Classic Edition will be available in the US.

The Innovation Hub was split into 3 panel discussions on assessing future health through AI-enabled tools, addressing patient workflow challenges through AI, and enhancing treatment through new technologies.

The PDUFA date for Brimochol PF is January 28, 2026.

While the study included a range of patients in a variety of stages of presbyopia, the change in crystalline lens and anterior segment geometry was most pronounced in emmetropic and myopic presbyopes.

Brimochol PF’s combination of brimonidine and carbachol produces a “pinhole effect,” which is intended to improve depth of focus and sharpen near and distant impact.
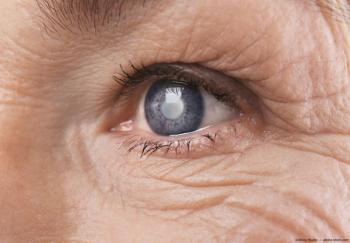
The lens has been introduced to select practices across the US, with broad commercial availability planned for in May.

The novel corrective eye drop can be prescribed through pharmacy partners BlinkRx or Medvantx.

The new lenses are designed for young presbyopes, ranging in age from their late 30s to mid-40s, who may be either existing spectacle wearers or non-wearers.

Understanding the precise formulation of pharmacologic agents for refractive correction for presbyopia.

Cecelia Koetting, OD, FAAO, DipABO, and Madeline Yung, MD, discussed emerging treatment and patient care options for those with presbyopia during CRU 2025.

The 6th World Congress of Optometry will be held during Saudi Society of Optometry’s 11th Optometry Conference and Exhibition.


Updates include enrollments for LYNX-2 pivotal Phase 3 and VEGA-3 pivotal Phase 3 trials, in addition to a US FDA Fast Track Designation granted for phentolamine 0.75% for the treatment of significant chronic night driving impairment in keratorefractive patients with reduced mesopic vision.

Attention to patients' near visual needs is warranted when choosing medications.

Two posters presented research conducted on the optical design used in MyDay Energys and Biofinity Energys.

The 3-arm, multicenter, randomized, double masked, safety and efficacy study successfully met pre-specified visual acuity primary endpoints with statistically significant near vision improvements recorded at all time points over 8 hours.
74% of participants dosed with LNZ100 achieved 3 or more lines of improvement 3 hours post-treatment.

The company previously released positive data results from the pivotal Phase 3 CLARITY study in April, with the NDA submitted in August of this year.


The TECNIS Odyssey IOL is a new full vision range IOL built on the TECNIS platform.