
The trial will evaluate the safety and effectiveness of the company's titratable glaucoma therapy system, designed to optimize IOP reduction in patients undergoing glaucoma surgery.

The trial will evaluate the safety and effectiveness of the company's titratable glaucoma therapy system, designed to optimize IOP reduction in patients undergoing glaucoma surgery.

This enables the initiation of the EYETAC phase II clinical trial (NCT07285070) in patients with non-infectious anterior uveitis (NIAU).

Glaukos noted under the updated labeling, physicians may now re-administer iDose TR more than once in patients who maintain a healthy cornea, as defined by corneal endothelial cell density parameters.

The study looked at 128 eyes in 64 patients before and after they had received the vaccine.

FDA authorizes compassionate use of urcosimod for neuropathic corneal pain, a condition that causes severe pain and sensitivity of the eyes, face, or head.

The Bimatoprost Drug Pad-IOL System is intended for the lowering of intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).

The investigational new drug (IND) application from Complement Therapeutics for CTx001 was previously approved by the FDA in October 2025.

The study evaluated thermal dynamics associated with CW-TSCPC and TLT using MicroPulse technology, such as temperature peak, exposure duration, and thermal spread in a simulated ciliary body using computer modeling.

NCX 470 is Nicox’s lead dual-mechanism bimatoprost eye drop for lowering IOP in open-angle glaucoma or ocular hypertension.

K8 is a member of a new class of inflammasome-inhibiting drugs called kamuvudines.

Aldeyra met with the FDA on December 12, 2025, in which the FDA requested the company submit the CSR from the reproxalap dry eye disease field trial to the NDA.

Xelafaslatide is a small-molecule Fas inhibitor designed to protect key retinal cells, including photoreceptors, from cell death.

Under the terms of the agreement, Iolyx Therapeutics has granted Théa exclusive worldwide development and commercialization rights, excluding Asia, to ILYX-002 for the treatment of ocular surface diseases.

HUC1-394 is a peptide-based eye drop for dry eyes being developed by the company.

This is the first submission for approval under the exclusive license and commercialization agreement for South Korea that was signed between LENZ and Lotus.

Cyclosporine ophthalmic emulsion 0.05% is a topical immunomodulator to increase tear production in patients whose tear production is thought to be reduced due to ocular inflammation associated with dry eye syndrome.

Additionally, the FDA approved a monthly dosing option for some patients who may benefit from resuming this schedule across approved indications.

The trial evaluated the safety, efficacy, and tolerability of AURN001 in patients with corneal edema secondary to corneal endothelial dysfunction.

The trial will evaluate the solution in treating significant, chronic night driving impairment in keratorefractive patients with reduced mesopic vision.

Outlook Therapeutics seeks FDA clarity on ONS-5010 after a complete response letter, aiming to address efficacy concerns for wet AMD treatment.

Naureen Haroon, OD, MPH, FAAO, founder of the newly formed WHSIG, said the group's primary aim is to educate the optometric community about why certain eye conditions disproportionately affect women.

FDA issues a complete response letter to Outlook Therapeutics for ONS-5010, citing insufficient evidence of effectiveness for wet AMD treatment.

The acquisition focuses on LayerBio's OcuRing-K technology, with plans to initiate the next clinical trial.

TearCare is associated with greater health utility over time but also resulted in significant cost savings compared with CsA.

The planned acquisition of STAAR includes the EVO family of lenses (EVO ICL) for vision correction for patients with moderate to high myopia with or without astigmatism.

The solution missed the primary end point of the trial, which was complete resolution of debris after 6 weeks of twice-daily dosing.

A Prescription Drug User Fee Act (PDUFA) target action date of December 16, 2025, was assigned by the FDA.

LumiThera’s PBM is the only device that has demonstrated meaningful vision improvement compared with baseline for people living with early to intermediate dry AMD.

Aldeyra resubmits NDA for reproxalap, aiming to address FDA concerns and demonstrate efficacy in treating dry eye disease symptoms.
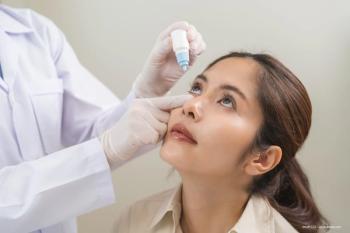
The generic formulation references Pred Forte, which is trademarked by Allergan.

Published: July 2nd 2024 | Updated:

Published: April 9th 2025 | Updated:

Published: July 8th 2025 | Updated:

Published: May 16th 2025 | Updated:

Published: August 2nd 2024 | Updated:

Published: August 6th 2025 | Updated: