
Justin Schweitzer, OD, FAAO, from Vance Thompson Vision, talked about his session at the IKA Symposium that discussed cross-linking in keratoconus treatment both now and looking toward the future.

Justin Schweitzer, OD, FAAO, from Vance Thompson Vision, talked about his session at the IKA Symposium that discussed cross-linking in keratoconus treatment both now and looking toward the future.

Barry Eiden, OD, FAAO, FSLS, President and Co-founder of IKA, talked about his session at the meeting centering around the pediatric prevalence of keratoconus, the importance of early diagnosis, and the impact this can have on practices.

Christine Sindt, OD, FAAO, Clinical Professor of Ophthalmology and Visual Sciences at the University of Iowa School of Medicine, talked about her session at the meeting focusing on higher order aberrations and the correction of complicated optics on scleral contact lenses.

Alan Glazier, OD, FAAO, talked about moderating a session discussing the education of the public on keratoconus, from screening to diagnosis, and the roles clinicians can take to assist in the patients' anxieties.

Andrew Morgenstern, OD, FAAO, FNAP, President and Co-founder of IKA, talked about his session at the meeting that discussed the prevalence of keratoconus in the pediatric population in the US.

Edmund Tsui, MD, details findings from a study that suggests that swept-source anterior segment OCT images by provide more objective ways to measure inflammation in the eye.

Michael Chaglasian, OD, details results from a study that assessed the new Monaco OCT's efficacy in glaucoma detection alongside ultra wideview imaging.

Emily Chew, MD, who won this year's Proctor Award, outlines her presentation on preparing for estimated doubling of age-related macular degeneration cases in the next 25 years at ARVO 2024.

Noel Brennan, MScOptom, PhD, overviews his ARVO 2024 presentation on reimagining the prediction of longer term efficacy numbers in data from pediatric trials evaluating myopia.

Deborah Ferrington, chief scientific officer at Doheny Eye Institute, details a session she gave on May 9 regarding mitochondria and how they change with health and in the diseased retina.

Giulia Corradetti, MD, discusses study findings that changes in microperimetry are highly localized and dependent on the OCT features.

Osamah Saeedi, MD, MS, details his ARVO 2024 presentation and the study finding's implications on monitoring and treating glaucoma.

The 2 FDA registration trials for perfluorohexyloctane resulted in scientifically significant results for improvement of mild and moderate symptoms of dry eye disease.

Neda Gioia, OD, CNS, FOWNS, CFMP, details recent findings on the efficacy of the NutriTears supplement on dry eye symptoms.

David A. Berntsen, OD, PhD, FAAO, talks through his 2024 ARVO presentation on the BLINK2 study and the findings on axial growth after discontinuing soft multifocal contact lens wear.

UPFs include fast food, energy drinks and soda, sweets such as chocolates and candies, and much more.
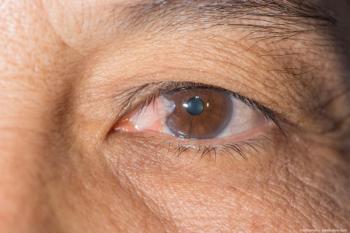
Pterygium is an ocular disease commonly referred to as “surfer’s eye,” which can result from exposure to high levels of UV radiation.

The Phase 2 trial tested whether inhibiting microglia with minocycline might help slow GA expansion and its corresponding vision loss.

The transient receptor potential melastatin 8 (TRPM8) are cold-sensitive thermoreceptors that play central role in tear film homeostasis.

LL-BMT1 is a novel, preservative-free, weekly drug-eluting contact lens created by the company’s proprietary 3D printing technology.

The event, being held at the El Conquistador Resort in Puerto Rico, is offering programs for ophthalmologists and optometrists.
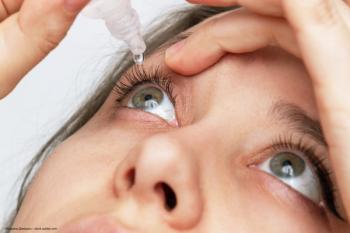
According to OKYO Pharma, the first-in-human, randomized, double-masked, placebo-controlled trial of OK-101 “established a clear and informed path for further development in Phase 3 registration trials.”

The TENEO excimer laser platform is intended for use for LASIK vision correction surgery for myopia and myopic astigmatism.
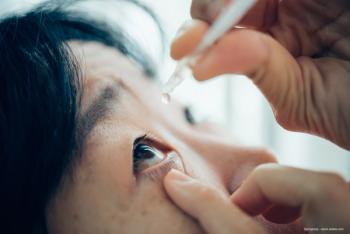
The revision is in response to multiple recalls for eye drops and instances of consumer injury and death in 2023.
A look back at some of the biggest stories in ophthalmology in 2023.

Eye care witnessed a transformative year with 11 FDA approvals. As the year concludes, there remains a robust pipeline of drugs, setting high expectations for continued advancements in ophthalmological care in 2024 and beyond.

TP-03 (lotilaner ophthalmic solution, 0.25%) was approved by the FDA in 2023 under the brand name Xdemvy for the treatment of Demodex blepharitis and is being evaluated as an investigational therapy for the treatment of Meibomian Gland Disease (MGD) in patients with Demodex mites.

A study surveying retina specialists found surprising results in therapy preference.

This is just one of many times the FDA has issued a warning to companies for the sale of unapproved ophthalmic products in 2023.
Aldeyra Therapeutics encounters a significant setback, with its stock value plummeting nearly 70%. The FDA indicates a potential Complete Response Letter (CRL) for reproxalap, a dry eye treatment, despite a scheduled Prescription Drug User Fee Act (PDUFA) date of November 23, 2023.