Martin David Harp, Editor, Ophthalmology Times
Articles by Martin David Harp, Editor, Ophthalmology Times

A groundbreaking study conducted by the International Agency for the Prevention of Blindness (IAPB) and Kevin Frick, PhD, from Johns Hopkins University reveals that prioritizing better eye health could inject a staggering $50.4 billion annually into the US economy.

Himalayan Cataract Project is celebrating World Sight Day by bringing attention to avoidable blindness globally to help individuals in developing countries retain and regain their sight.

Ocuphire Pharma and Viatris developed the drug together for the reversal of pharmacologically-induced mydriasis (RM) produced by adrenergic agonist or parasympatholytic agents.

The theme calls on employers, insurers, and policy makers to consider how they can contribute to improved access to vision and eye health care.
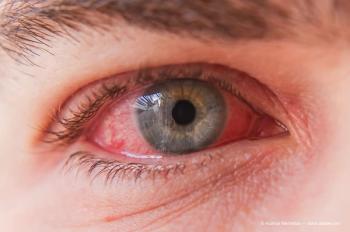
The week of September 18-24 will be dedicated to educating the public on IEDs such as uveitis, keratitis, conjunctivitis and more.

Walgreens Boots Allianc Inc. and CVS Health are among the companies the FDA sent letters to for violating federal law.
The organization says to stop using Dr. Berne’s and LightEyez MSM drops immediately due to bacterial contamination, fungal contamination, or both.

Canadian and Australian ophthalmologists and optometrists are looking ahead at the impact increased wildfire smoke can have on the ocular surface.

The company has completed evaluation on candidates and selected a single drug candidate to move forward into clinical trials.

CT1812 is an experimental, oral therapy for the treatment of geographic atrophy secondary to dry age-related macular edema.

Horizon Therapeutics announced the Brazilian Health Regulatory Agency (ANVISA) has approved TEPEZZA as the first and only medicine approved in Brazil for treatment of thyroid eye disease.

The company announced the Complete Letter Response from the FDA for the Biologics License Application for aflibercept 8 mg is “solely due to an ongoing review of inspection findings at a third-party filler."

The organization aims to educate viewers of dangers and safety precautions ahead of the eclipses.

The agreement includes rights to develop, manufacture and commercialize NOV03 in Japan for the treatment of dry eye disease.

EverPatch is described by the company as the world's first non-degradable, synthetic tissue substitute for ophthalmic surgery.

The trial is evaluating Oxurion’s novel plasma kallikrein (PKal) inhibitor THR-149 as a potential treatment for DME patients who respond suboptimally to anti-VEGF therapy.

NVK002 is a proprietary, investigational, preservative-free eye drop administered nightly and intended for patients ages 3 to 17.

The day helps to bring focus and awareness to risk factors for early vision loss associated with smoking.
The two companies have announced an exclusive agreement for Polifarma to commercialize AVT06, the proposed biosimilar to Eylea, in Turkey.

John Berdahl, MD, discussed the Aurion clinical trials of endothelial cell therapy injection at the 2023 ASCRS annual meeting in San Diego.

Anthony Wallace, Vice President and General Manager of the US Surgical Business at Bausch + Lomb, discussed the introduction of SeeLuma, a fully digital surgical visualization platform, and more at the 2023 ASCRS annual meeting in San Diego.

ASCRS 2023: Analysis in two subgroups of routine cataract surgery
ByEdward Hu, MD, PhD,Sydney M Crago, Editor, Modern Retina,Sheryl Stevenson, Eye Care Group Editorial Director,Martin David Harp, Editor, Ophthalmology Times Edward Hu, MD, PhD, discussed his paper on the single surgeon retrospective comparative analysis of 517 eyes of two subgroups of routine cataract surgery at the 2023 ASCRS annual meeting in San Diego.
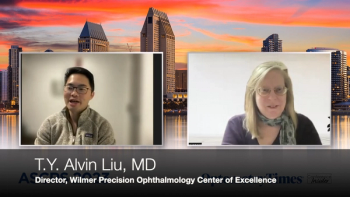
Alvin Liu, MD, sat down with Sheryl Stevenson, Group Editorial Director, Ophthalmology Times®, to discuss his presentation on deep learning and 3D OCT at the ASCRS annual meeting in San Diego.

Deborah Ferrington, PhD, shares a brief overview of her presentation on identifying the mechanism responsible for the death of the retinal pigment epithelium.
ARVO 2023: Lexitas presents its modified NEI scale
ByAndrew Pucker, OD, MS, PhD, FAAO,George Magrath, MD,Martin David Harp, Editor, Ophthalmology Times,David Hutton, Managing Editor, Ophthalmology Times Andrew Pucker, OD, MS, PhD, FAAO, and George Magrath, MD, discuss Lexitas Pharma Services' work on modification of the National Eye Institute's corneal fluorescein staining scale.

Mark Bullimore, PhD, MCOptom, shares a brief overview of her presentation on myopia management by slowing progression with low concentration atropine.

Dirty equipment and sterilization issues are among the many violations found by the FDA.
Kristen Ingenito, MBA, gives key takeaways from her G360 presentation, "A bird’s eye view of innovation."
Shan Lin, MD, gives key takeaways from his G360 presentation, "The latest in IOLs: When and how to use them?"
Robert Stamper, MD, gives key takeaways from his G360 presentation, "Home tonometry: Worth the pressure?"































