Geographic Atrophy
Latest News
Video Series
Latest Videos
Podcasts
CME Content
More News

Knowing what’s on the market for AMD and GA aids in preserving vision.

K8 is a member of a new class of inflammasome-inhibiting drugs called kamuvudines.

Xelafaslatide is a small-molecule Fas inhibitor designed to protect key retinal cells, including photoreceptors, from cell death.
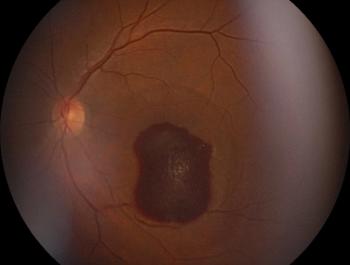
In the study, authors investigated the natural history of GA lesion incidence rates and analyzed potential risk factors for faster incidence of GA lesions.

Fovea-sparing, multifocal, and bilateral lesions exhibited the fastest growth rates.

The lesion area growth was reduced by more than 50% in its first human clinical trial of K8.
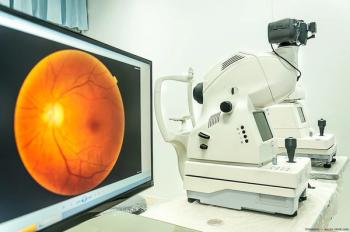
Researchers conducted this study to determine how often and why spontaneous soft drusen regresses without atrophy in patients who had intermediate or atrophic age-related macular degeneration.
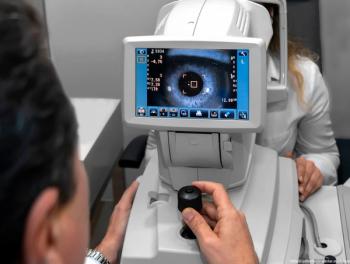
Gyrate atrophy of the choroid and retina is a form of choroidal sclerosis and is a rare, progressive, autosomal recessive, inherited genetic disease that primarily affects the ocular tissue.

LumiThera’s PBM is the only device that has demonstrated meaningful vision improvement compared with baseline for people living with early to intermediate dry AMD.

A study reveals a genetic link between instant coffee consumption and increased risk of dry age-related macular degeneration, urging caution for high-risk individuals.

The LMDD Eye Health Scorecard specifically evaluated insurance coverage for treatments addressing GA, neovascular AMD, and thyroid eye disease.
The results of an investigation into iRORA identified a wide spectrum of fundus autofluorescence patterns that corresponded with iRORA lesions and that those patterns were associated with conversion to cRORA over time.

Valeda met the primary end point in the US LIGHTSIDE III trial, improving the best corrected visual acuity in patients for 24 months of >5 letters or equivalent to 1 line improvement on the eye chart, according to the company.
Topline results from the MAGNIFY phase 2 clinical trial of oral zervimesine show 28.6% slower geographic atrophy lesion growth compared with placebo.

Boehringer Ingelheim announced that the phase 2 clinical studies will investigate a potential first-in-class oral compound and a highly specific antibody fragment for geographic atrophy.

With the acquisition, Topcon Healthcare also assumes RetInSight’s AI algorithms that analyze retinal images to detect and monitor disease.


The first 6 patients in the study had an average improvement of 14.9 letters in early treatment diabetic retinopathy study (ETDRS) standard vision tests within 4 to 6 months post transplantation.

Rachelle Lin, OD, MS, FAAO, and Quan Đông Nguyễn, MD, MSc, presented available treatment options and what is coming down the pipeline for retinal conditions.

Results from a recent study led researchers to advise clinicians of the potential for dystrophies that mimic age-related macular degeneration and other atrophic macular pathologies to be incorrectly diagnosed, which can impact treatment.

This follows successful Phase 1 results, which demonstrated a favorable safety profile for BI 771716 across both single and multiple intravitreal doses.

OCU410 is a novel multifunctional modifier gene therapy candidate that targets multiple pathways associated with GA.

The company also reported a positive outcome of an analysis of masked data from its ongoing MAGNIFY Phase 2 trial for zervimesine in adults with GA.
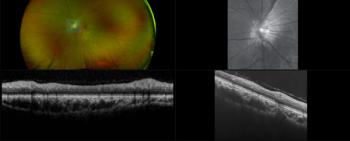
MonacoPro, the next evolution of Monaco from Optos, retains the powerful ultra-widefield SLO and spectral domain imaging while adding additional key product features.