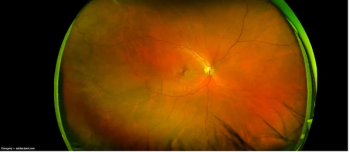
Clinical benefit of complement inhibition has been demonstrated in clinical trials, but there are possible consequences to consider.

Clinical benefit of complement inhibition has been demonstrated in clinical trials, but there are possible consequences to consider.
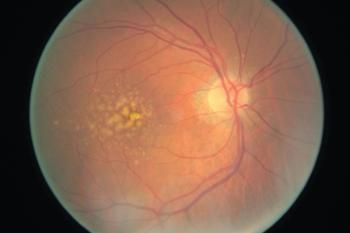
How to diagnose and monitor progression.
Diagnose, monitor, comanage—optometrists support patients throughout all stages of the disease.

David S. Boyer, MD, shares highlights from the symposium on advances in the geographic atrophy space, which he presented during the 17th annual Controversies in Modern Eye Care meeting.
The GA Won’t Wait campaign is designed to help older adults and their families understand and recognize the symptoms of this progressive and irreversible disease.

Catch up on what happened in optometry during the week of April 17-April 21.

One way to advance the current understanding of GA is to study how disease progression varies among related individuals.

A look back on what happened in optometry during the week of April 10-April 14.
Pegcetacoplan’s recent FDA approval creates an option for patients with dry AMD.

A look back on what happened in optometry during the week of April 3-April 7.
Euin Cheong, OD, weighs in on the Syfvore approval for geographic atrophy and how optometrists should participate in geographic atrophy care.
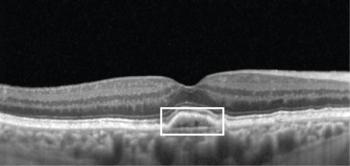
Imaging can elucidate visual prognosis and disease progression risk.
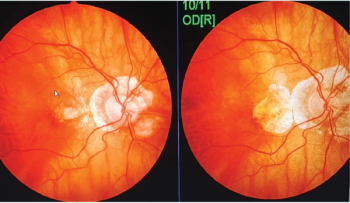
These disorders account for most of the vision loss in working-age and elderly Americans.

Mohammed Rafieetary, OD, shares key takeaways from his SECO 2023 presentation, "Advancing the understanding of geographic atrophy."
Following the submission of the 24-month phase 3 data in November 2022, Apellis received FDA approval for intravitreal pegcetacoplan (SYFOVRE) to treat geographic atrophy secondary to AMD.
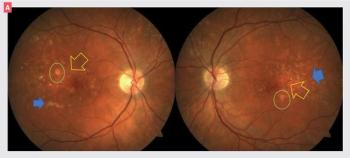
Look out for at-risk patients and prepare to establish treatment protocols.
The submission of 24-month efficacy data from DERBY and OAKS is classified as a Major Amendment to the New Drug Application, which delays the PDUFA target action date until February 2023.
Jessica Steen, OD, FAAO, shares highlights from her VEW 2022 presentation, "Ophthalmic therapeutics update."
Latest data from phase 3 studies finds increased effects over time with intravitreal pegcetacoplan for geographic atrophy secondary to age-related macular degeneration.

If approved, pegcetacoplan—an investigational, targeted C3 therapy—will be the first-ever treatment for GA.
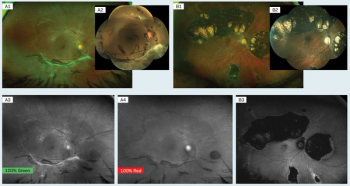
Advancements enable better diagnosis, treatment of patients.

A decision by the FDA is expected in August 2022.
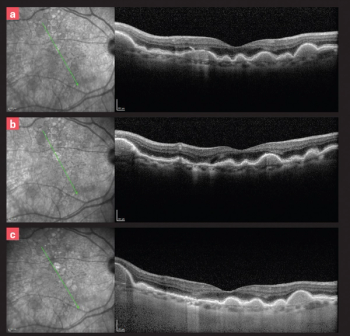
A look at the latest developments in retinal disease, including clinical trials and advanced therapies.

New detailed, longer-term findings were presented at the 2022 ARVO meeting.