Geographic Atrophy
Latest News
SeaBeLife announces preclinical efficacy results for SBL03 topical ophthalmic gel
Latest Videos

CME Content
More News

Whitecap is currently developing 2 therapies for potential use in glaucoma and geographic atrophy.

The application was refiled following a December 20, 2024, meeting between the US Food and Drug Administration (FDA) and Astellas.

The potential gene therapy candidate is being evaluated for geographic atrophy.

From new treatments on the horizon for macular degeneration to strengthening comanagement ties, optometrists cite a lot to be excited about in the coming year.

This year was brimming with advancements in eye care.

The global survey revealed similar challenges for individuals with unilateral and bilateral geographic atrophy.

EnVision Summit optometry faculty Sherrol Reynolds, OD, FAAO; Katie Rachon, OD, FAAO, Dipl ABO; Ashley Wallace-Tucker, OD, FAAO, FSLS, Dipl ABO; Jessica Steen, OD, FAAO, Dipl ABO; and Cecelia Koetting, OD, FAAO, Dipl ABO; express excitement for the upcoming conference and why optometrists should attend.

A few optometrists share which innovations in optometry they were most excited about in 2024.
Brush up your geographic atrophy knowledge with our 2024 content highlights.

The organization will provide resources for spreading awareness and educating patients and care givers.

The FDA comments in the CRL relate to proposed labelling language, not safety, Astellas said in a press release.
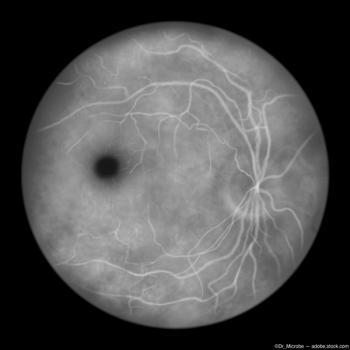
The data showed a reduction in the GA lesion growth rate of 15.3% versus placebo from 6 to 24 months and a slowing in GA growth rate of 13.4% from baseline to 24 months.
Study suggests that small hyperreflective retinal foci may represent local neuroretinal inflammation
Study authors defined hyperreflective retinal foci as “isolated punctiform elements of small dimensions, 30 μm or smaller, with intermediate reflectivity (similar to that of the nerve fiber layer) and without a shadow cone.”

By identifying progressive GA earlier, primary eye care providers can make timely referrals and save patients’ visual function.

Understanding the latest innovative technologies.

Comanagement is a key component for treatment of disease.

A new analysis by researchers at the National Institutes of Health shows the benefit of taking AREDS2 formula in late AMD.

Utilizing artificial intelligence may provide feasibility assessments, data-driven protocol design, and cost reduction opportunities.

Apellis will seek re-examination from the CHMP following the opinion, according to a news release.
The company expects enrollment of the GAZE phase 1, open-label clinical trial, to begin in the second half of 2024.

Positive data from the GALE long-term extension study showed patients developed fewer new scotomatous points with 36 months of both continuous monthly and every-other-month treatment.

Real-world insights can clear the path to trends and strategies to meet patients’ needs.

The company reported that statistically significant data was found supporting ANX007’s ability to provide vision loss protection in patients with geographic atrophy.

The non-randomized, open label, safety and efficacy study will inject a sustained release implant in 5 participants.