The good and the bad of telemedicine in the care of our patients with diabetes
Is telemedicine through retinal images a solution to earlier intervention and increased access to care? Fortunately—and unfortunately—yes.
(Image credit: Adobe Stock/©volha_r)

More than 130 million adults in the US have diabetes or prediabetes,* and approximately one-third of them will develop retinopathy in their lifetime.1 Vision-threatening forms of diabetic retinopathy are a leading cause of blindness in working-aged adults and are estimated to occur in 5% of patients with diabetes. It has been shown that those with the highest risk of being in that 5% are often patients with job insecurity, low household income, transportation insecurity, inadequate insurance coverage, or other social determinants of health.2 Preventive measures include proper blood glucose control, systemic care for comorbidities including hypertension and hyperlipidemia, and early treatment.
However, in many cases, diabetic retinopathy is diagnosed far later than what would be ideal for the best visual outcomes. Other important steps (eg, patient education, facilitation of lifestyle modifications, more frequent follow-up visits, and advanced retinal imaging) are not allowed the chance to help aid in prevention. Is telemedicine through retinal images a solution to earlier intervention and increased access to care? Fortunately—and unfortunately—yes. The history of telemedicine in optometry is rich, with roots back to the 1970s and a primary focus on retinal photography for those with inadequate access to care. The frequency of diabetic retinal screenings has markedly increased in the past decade.
The good
Screening for diabetic retinopathy through retinal photography is aimed at serving patients who may otherwise not have access to eye care, catching diabetic retinopathy and other conditions for earlier detection and treatment to avoid permanent vision loss. Earlier diagnosis and prevention of more serious disease also has the potential to ease the financial burden on health care costs.3
As few as 35% of patients with diabetes are reported to be adherent to the recommendation of an annual dilated eye examination,4,5 which is often related to logistical barriers such as transportation, lack of insurance, and other social determinants of health. Given the increasing prevalence of diabetic retinopathy, as well as poor access to eye care for so many patients at the highest risk for vision loss, telemedicine through diabetic retinal imaging serves to bridge the gap.2 Incorporating a retinal photograph during a primary care visit is logical, efficient, cost-effective, and can yield results.6-9
The bad
Particularly when you take full advantage of today’s technology of ultrawide field retinal imaging (UWFI), special filters, and increased resolution, a retinal photograph can often provide a thorough screening of retinal health. However, the retinal photograph is no more than that: a retinal photograph. All other aspects of the eye’s health are unknown to the provider who ordered the photo, the eye care specialist who interpreted the photo, and to the patient who is almost certainly under the impression that they had an eye exam.
Unfortunately—and in part due to insurance companies allowing retinal photographs to be perceived as synonymous with a retinal exam for patients with diabetes—there is a major disconnect among patients and health care providers alike when it comes to what the photo is providing.
Federally incentivized quality measures define a diabetic retinal exam as a “dilated retinal exam or photo,” adding insult to injury with patient and doctor misinterpretation of the photo’s value. A screening retinal photo will likely be successful in screening for the absence or presence and degree of diabetic retinopathy,10,11 but it will fail to screen for reduced vision, increased IOP, iris melanoma, peripheral retinal detachment, and many other pathologies. Is it a replacement for a comprehensive eye exam? No; but it is accessible, cost-effective, and an improvement over no eye exam at all. If the retinal photograph did provide a screening for a patient without access to eye care, particularly if the screening photograph leads to a referral of any kind, the outcome is a success.
The process
The retinal photograph is a form of asynchronous telemedicine; it is taken on-site, typically in a primary care or endocrinology setting, then later interpreted remotely by a designated trained analyst. The individual who interprets the photograph then provides the ordering physician with a report and recommendation. The objective of retinal photography for these clinics is primarily focused on providing patients with the care they need and, in doing so, often allows them to meet federal quality measures, which increases federal payer reimbursements.
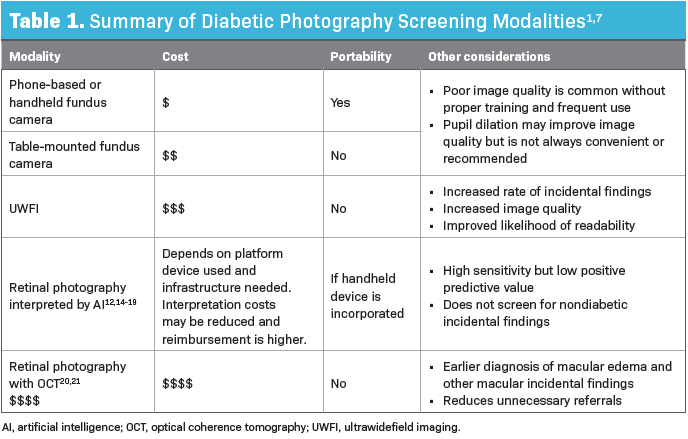
A variety of modalities and technologies exist, providing primary care clinics with many options to consider (Table 1). Ease of use, portability, cost, the potential need for mydriatic eye drops, and image quality are all considerations for clinics that are purchasing a camera. For those of us in the eye care business, it is clear that UWFI s often preferred to central retinal photography when it comes to screening, but we are familiar with the exceptions to that and are confident with which clinical circumstances might benefit with one over the other.
Handheld and table-mounted cameras used by staff who only occasionally take retinal photos can often lead to poor image quality. Although pupillary dilation often provides improved image quality for retinal photography,12 the use of mydriatic agents is, understandably, an unpopular option among primary care clinics and patients alike.
On the other hand. Ultrawide field cameras can be much more forgiving. Retinal image readability is a major limiter in success, with more than half being attributable to operator error.13 Optometrists also know well that staff training can make or break image quality, regardless of the imaging device. However, providers and clinics pursuing retinal photography as a means of screening for diabetic retinopathy do not typically realize the advantages and disadvantages of these features and may benefit from guidance from their local eye care provider before purchasing a device. Unfortunately, the eye care provider is often not involved in this step.

Return on financial investment is a challenge, given low reimbursement rates for imaging procedures, but this has improved significantly since the COVID-19 pandemic, as the value and realized need for telemedicine in many aspects of health care has increased. Quality reporting and billable Current Procedural Terminology (CPT) codes support the retinal screening photo annually until retinopathy is seen, at which point an in-person eye exam is recommended. A summary of current CPT codes pertaining to screening retinal imaging are listed in Table 2.8,9 Per federal definitions, insurance companies reimburse the same amount for retinal photography, regardless of the device used, making it difficult to justify increased expense on the front end. Although the UWFI option offers much more information than those that photograph the central 30º or 45º of the retina, it is much more expensive to purchase, takes up more physical space, and offers no additional reimbursement.
To summarize, CPT codes 92227 through 92229 are used for screening patients with diabetes without previously noted retinopathy. These codes can be billed annually under most payers, with some exceptions; for example, a patient with type 1 diabetes must have the condition for 5 years before the photograph is covered. Once diabetic retinopathy is noted, the use of these codes is not applicable, as the care is no longer screening in nature. At the point in which retinopathy is noted, the patient should transition to in-person care. Codes 92227 and 92228 are similar, except for who interprets the image and provides the report. CPT code 92227 is used when the image is interpreted and reported on by trained clinical staff, whereas 92228 is appropriate when a trained optometrist or ophthalmologist does so. CPT code 92229 specifically refers to the use of artificial intelligence (AI) for interpretation. CPT code 92250 is used for fundus photography with interpretation and report, often used clinically by eye care providers during an in-person assessment. It is typically used to document a retinal finding once an in-person retinal exam has revealed a condition to document; however, some payers may reimburse for 92250 for telemedicine-based screening. Familiarity of the primary care provider (PCP) with contracted insurance plans is of utmost importance to comply with current coding usage and restrictions. As this is fairly new territory, codes and rules have undergone frequent change.
Retinal image interpretation using AI
Since 2018, the FDA has cleared a handful of AI algorithms for the detection of diabetic retinopathy. These systems, paired with cameras and trained analysts or eye care providers as indicated, have shown promise for improved efficiency and cost-effectiveness for diabetic retinopathy screenings. Studies have shown that AI-based image analyses can produce fairly accurate results, with sensitivity reported to be 87% to 98% and specificity being 84% to 93%. Importantly, the positive predictive value (PPV) has been reported to be as low as 30% to 40%, leading to some unnecessary referrals. However, PPV does improve with increasing severity of retinopathy. Additionally, AI-based systems do not screen for other pathology, such as glaucoma or retinal detachment.12,14-19
Remote OCT screening
Optical coherence tomography (OCT) may also be incorporated into telemedicine programs for diabetic retinal screening, although cost, space, and patient flow are limiters. Although the Centers for Medicare and Medicaid Services has assigned a temporary code for remote OCT with interpretation (Table 2),8,9 there is currently no reimbursement. As technology emerges and image resolution increases, eye care professionals are increasingly dependent on OCT to allow for accurate diagnoses of diabetic macular edema (DME). In particular, OCT helps to detect subtle but treatable center-involved DME earlier, and likewise may prevent unnecessary referrals for those cases that do not require treatment. Retinal photographs alone may fall short.20 When OCT was compared with UWFI, OCT was found to not only have a much lower false positive rate for DME (reducing unneeded referrals) but also a higher sensitivity to detection of DME.21
Eye care partnerships with the PCP
Success in diabetic retinal screenings does not stop at accurate image interpretation but includes the critical step of proper referral, patient education, and follow-up. PCPs and others ordering the photos may benefit from guidance on local referral options, including details of the specific type of referral, is preferred (eg, optometrist, retina specialist).
Retinal images captured for the purpose of diabetic retinopathy screenings are interpreted remotely by a designated optometrist, ophthalmologist, or by AI. It is typical for the clinic, and owner of the camera, to bill for and collect the full reimbursement for the retinal photograph and interpretation. The clinic then pays an agreed-upon interpretation fee to the eye care provider. Some companies that sell the cameras also manage the interpretation and payment to the image interpreter.
Typically, the eye care provider interpreting the images has no other relationship with the clinic for which images are being interpreted. However, there are a variety of exceptions to these typical arrangements. In some less-common circumstances, clinics that are utilizing diabetic retinal screening photos have a direct relationship with the eye care provider who is interpreting the photos. In these cases, there is a unique opportunity for collaboration between the eye care provider and the PCP, allowing for enhanced care and closed loops for the patient. These relationships may also be less at risk for the frequent misunderstanding between eye exams and retinal photos, given that a platform for staff and provider education opportunities may exist.

Image interpretation often includes a brief description and grade of any noted retinopathy, including presence or absence of macular edema, and recommended referral urgency. Some modalities allow the interpreting eye care provider to ad-lib regarding pertinent considerations, incidental findings, or referral details, whereas others may offer limited flexibility. Examples of interpretation and recommendation verbiage are listed in Table 3. Note that these recommendations may be considered fairly conservative or referral heavy. Timing and details of referrals may vary based on findings and any known risk factors. Direct connection with the patient’s medical history and/or PCP can contribute to more effective referral decisions and often avoid unnecessary referrals.
As always, referral decisions should be patient focused, with an emphasis on patient convenience, insurance coverage, preference, and best care. Prearranged referral relationships are not appropriate and may insinuate provider self-interest, as well as violate the Anti-Kickback Statute.22 Referrals that are urgent in nature may need a special process beyond the cloud-based communication (eg, phone call or secure text) to ensure communication and prompt action.
Optometry’s role
As a profession, optometry has no choice but to embrace the concept of screening for diabetic retinopathy by telemedicine and will ideally maintain a seat at the table. The practice is strong and is and growing stronger as technology advances and infrastructures are enhanced. Optometrists with direct involvement may have opportunities to improve referral patterns and contribute to patient and health care provider education. Although these systems are far from perfect, the goal is to serve patients who need care most. Evidence supports the success of improving health care outcomes and reducing the financial burden of health care costs.
*The original article incorrectly cited that more than 130 million adults in the US have diabetes when in actuality, 38.4 million people have diabetes and 97.6 million people have prediabetes. The article was updated for corrected context on November 16, 2023.
References
1. Lundeen EA, Burke-Conte Z, Rein DB, et al. Prevalence of diabetic retinopathy in the US in 2021. JAMA Ophthalmol. 2023;141(8):747-754. doi:10.1001/jamaophthalmol.2023.2289
2. Chan AX, McDermott Iv JJ, Lee TC, et al. Associations between healthcare utilization and access and diabetic retinopathy complications using All of Us nationwide survey data. PLoS One. 2022;17(6):e0269231. doi:10.1371/journal.pone.0269231
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
4. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412-418. doi:10.2337/dc16-2641
5. Paz SH, Varma R, Klein R, Wu J, Azen SP; Los Angeles Latino Eye Study Group. Noncompliance with vision care guidelines in Latinos with type 2 diabetes mellitus: the Los Angeles Latino Eye Study. Ophthalmology. 2006;113(8):1372-1377. doi:10.1016/j.ophtha.2006.04.018
6. Das S, Kuht HJ, De Silva I, et al. Feasibility and clinical utility of handheld fundus cameras for retinal imaging. Eye (Lond). 2023;37(2):274-279. doi:10.1038/s41433-021-01926-y
7. Rathi S, Tsui E, Mehta N, Zahid S, Schuman JS. The current state of teleophthalmology in the United States. Ophthalmology. 2017;124(12):1729-1734. doi:10.1016/j.ophtha.2017.05.026
8. Shaver J. The state of telehealth before and after the COVID-19 pandemic. Prim Care. 2022;49(4):517-530. doi:10.1016/j.pop.2022.04.002
9. Lee SC, Lieng MK, Alber S, Mehta N, Emami-Naeini P, Yiu G. Trends in remote retinal imaging utilization and payments in the United States. Ophthalmology. 2022;129(3):354-357. doi:10.1016/j.ophtha.2021.10.010
10. Ullah W, Pathan SK, Panchal A, et al. Cost-effectiveness and diagnostic accuracy of telemedicine in macular disease and diabetic retinopathy: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(25):e20306. doi:10.1097/MD.0000000000020306
11. Liu Y, Rajamanickam VP, Parikh RS, et al. Diabetic retinopathy assessment variability among eye care providers in an urban teleophthalmology program. Telemed J E Health. 2019;25(4):301-308. doi:10.1089/tmj.2018.0019
12. Mehra AA, Softing A, Guner MK, Hodge DO, Barkmeier AJ. Diabetic retinopathy telemedicine outcomes with artificial intelligence-based image analysis, reflex dilation, and image overread. Am J Ophthalmol. 2022;244:125-132. doi:10.1016/j.ajo.2022.08.008
13. Knezevich FP III, Zimmer-Galler I, Zeimer R. Reasons for unreadable fundus images obtained by telemedicine during large scale diabetic retinopathy screening in the primary care environment. Invest Ophthalmol Vis Sci. 2009;50(13):3269.
14. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. doi:10.1038/s41746-018-0040-6
15. Verbraak FD, Abramoff MD, Bausch GCF, et al. Diagnostic accuracy of a device for the automated detection of diabetic retinopathy in a primary care setting. Diabetes Care. 2019;42(4):651-656. doi:10.2337/dc18-0148
16. Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020;8(4):337-347. doi:10.1016/S2213-8587(19)30411-5
17. Gunasekeran DV, Ting DSW, Tan GSW, Wong TY. Artificial intelligence for diabetic retinopathy screening, prediction and management. Curr Opin Ophthalmol. 2020;31(5):357-365. doi:10.1097/ICU.0000000000000693
18. Land MR, Patel PA, Bui T, et al. Examining the role of telemedicine in diabetic retinopathy. J Clin Med. 2023;12(10):3537. doi:10.3390/jcm12103537
19. Nakayama LF, Zago Ribeiro L, Novaes F, et al. Artificial intelligence for telemedicine diabetic retinopathy screening: a review. Ann Med. 2023;55(2):2258149. doi:10.1080/07853890.2023.2258149
20. Wong RL, Tsang CW, Wong DS, et al. Are we making good use of our public resources? the false-positive rate of screening by fundus photography for diabetic macular oedema. Hong Kong Med J. 2017;23(4):356-364.doi:10.12809/hkmj166078
21. Aiello LP, Jacoba CMP, Ashraf M, et al. Integrating macular optical coherence tomography with ultrawide field imaging in a diabetic retinopathy telemedicine program using a single device. Retina. 2023;10.1097/IAE.0000000000003883. doi:10.1097/IAE.0000000000003883
22. Fraud & abuse laws. US Department of Health and Human Services. https://oig.hhs.gov/compliance/physician-education/fraud-abuse-laws/#:~:text=The%20AKS%20is%20a%20criminal,for%20Medicare%20or%20Medicaid%20patients
23. Maturi RK, Glassman AR, Josic K, et al; DRCR Retina Network. Four-year visual outcomes in the protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA. 2023;329(5):376-385. doi:10.1001/jama.2022.25029
24. Glassman AR, Baker CW, Beaulieu WT, et al; DRCR Retina Network. Assessment of the DRCR Retina Network approach to management with initial observation for eyes with center-involved diabetic macular edema and good visual acuity: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2020;138(4):341-349. doi:10.1001/jamaophthalmol.2019.6035
