Case review of challenging ocular surface disease
A comprehensive approach to treatment is critical to help patients with comprehensive disease.

ODs are now able to provide a higher level of care to patients with significant ocular surface disease along with comorbid ocuar and systemic disease. With increased awareness of OSD and more attention to this unmet need, patient outcomes are improving.
Ocular surface inflammation and tear film dysfunction can go undetected, undertreated, or untreated for many patients. In most cases, uncontrolled inflammation is the major effect of multiple factors that drive a continuum of negative ocular surface changes. These include desiccation, corneal haze, and neovascularization-and they produce a myriad of symptoms, some of which are understandable and others that are more difficult to link to the clinical picture.
Interrupting this continuum of ocular surface compromise is, of course, more difficult the later along the continuum the intervention begins.1 Treating the ocular surface disease (OSD) patient early and monitoring response to treatment frequently is an important part of preservation of the ocular surface.1
Eyecare practitioners now have multiple, effective treatments and procedures they can employ in order to protect the ocular surface and beign to restore homeostasis. A comprehensive approach to ocular surface disease treatment is critical because patients struggling with symptoms are not likely to make all of the connections between factors which play a significant role in the dysregulation of the lacrimal functional unit.2,3 Of special interest in the area of new treatments is clinical research on growth factors and the restorative role they play.4-8
Related: Homeostasis is the Holy Grail in dry eye disease
The first paradigm shift in understanding OSD involved the importance of evaporative changes to the ocular surface. Since this pivotal step in understanding of the disease, ODs continue to uncover major inflammatory constituents that promote disease progression.9 The impact of chronic inflammation on corneal sensation, neuropathic pain, and the mechanisms involved in central sensitization which lead to various levels of pain in OSD is also now better understood.10
Clinical research and progress in the treatment of ocular surface disease is critical and serves a great need. The overall prevalence of dry eye disease in the U.S. is 40 million.11-14 Longstanding research has revealed the link among OSD, hormonal changes in female patients, and age.11-14 It is interesting to note that in addition to these well-known connections, OSD is an important differential in younger patients.14 Among multiple factors, perhaps the main reason for this development is the simple act of a proper blink. A better understanding of this multifactorial disease (that ODs often refer to by one of its features, “dry eye”) is paving the way for a more comprehensive picture of each case and better patient outcomes.
Below are two cases of patients in my clinic that highlight useful treatments in OSD.
Case 1: Sjögren’s dry eye
The patient’s pertinent history includes:
- 36-year-old Caucasian female
- Sjogren’s syndrome dry eye
- Excellent Snellen visual acuity: 20/20 OD, OS
- 8+ hours a day of computer work (architect)
- 400 mg hydroxychloroquine (HCQ) po qd
- Seasonal allergies
- Active lifestyle with outdoor activities
- Excellent patient compliance and reliability
- Timely follow-up and compliance with rheumatology
Reason for clinic visit were sharp, irritating pain in the left eye, which oke her up in the middle of the night. She was experiencing significant dryness, redness, and itching and finding it difficult to get through a day of work. The patient was also concerned about long-term impact of Sjögren’s syndrome dry eye (SSDE).
Related: Why patient occupation matters with dry eye disease
- Pertinent past ccular history includes:
- Meibomian gland dysfunction
- Evaporative dry eye and aqueous deficiency
- Blepharitis
- No history of chemical injury
- No smoking
- Currently using cyclosporin bid and 40 percent autologous serum qid OU
- Glaucoma suspect; need for intermittent use of steroid eyedrops during flare ups
Related: Dry eye in the digital age
Clinical exam findings:
- 20/25+ OD, OS
- Immediate tear break up OD, OS
- Incomplete blink OD, OS
- 2+ eyelid telangiectasia OD, OS
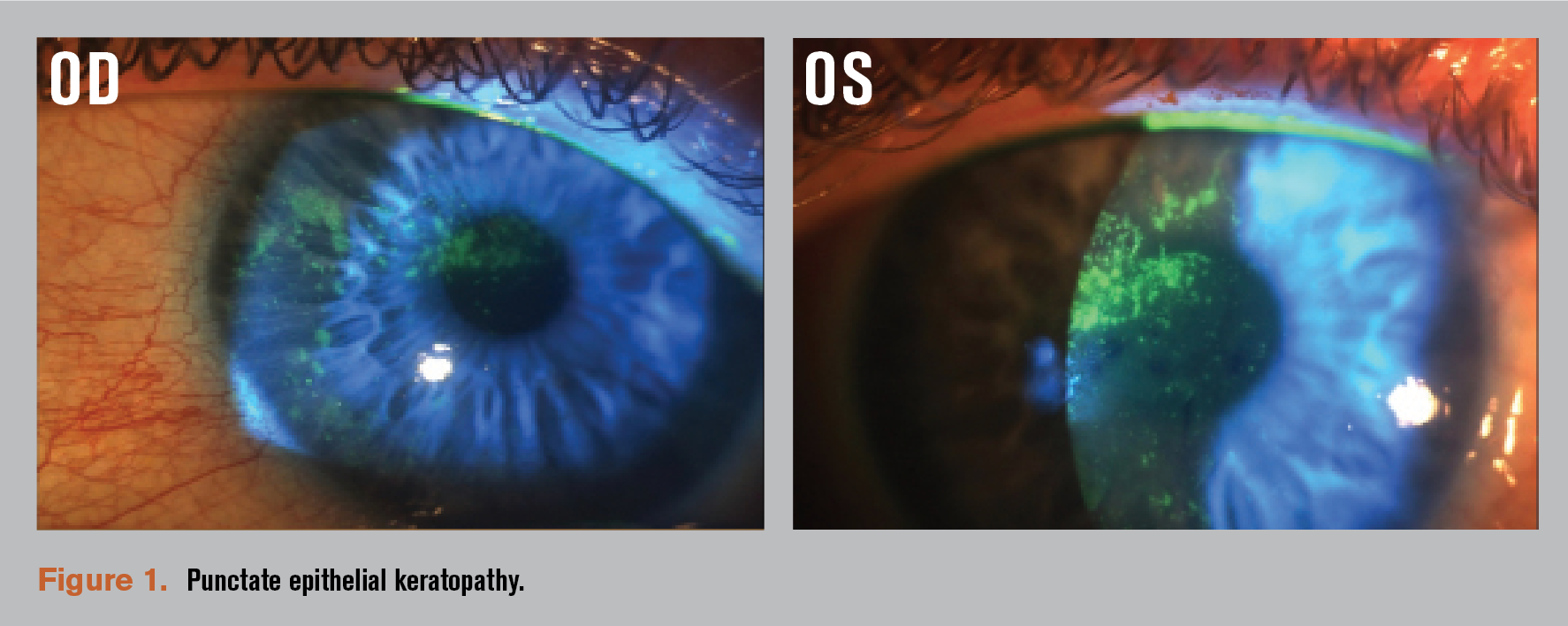
- Punctate epithelial keratopathy (PEK) involving the visual axis OS>OD (Figure 1).
- Conjunctival injection 3+ OS, 2+ OD
- Strong positive InflammaDry (Quidel) OD, OS
- Osmolarity: 330 OD, 339 OS
- Minimal secretion/meibomian glands yielding lipid (MGYL) with cotton-tipped expression OD, OS
- No significant eyelid malposition OD, OS
- Open puncta OD, OS
Case 1 discussion
This case presents several challenges and important considerations. Let’s start with one that might have a smaller spotlight on it and therefore can be more easily missed: retinopathy due to HCQ use in patients with Sjögren’s disease and other inflammatory conditions like rheumatoid arthritis and lupus.15
Although the majority of the evaluation and management of this patient centers around ocular surface disease, also carefully consider possible retinal toxicity from HCQ use. These patients can maintain excellent central vision in spite of significant HCQ retinopathy due to the concept of “foveal resistance” and preservation of the sub-foveal retina.15 In this case, the patient did not have maculopathy secondary to HCQ use.
Another important consideration is intraocular pressure (IOP) and optic nerve monitoring. The majority of the patients with an underlying systemic inflammatory condition will need a short-term treatment of steroid eyedrops and/or oral steroid. This is an appropriate treatment for these patients during flare-ups. As these treatment patterns are repeated, it is important to monitor the retinal nerve fiber layer and visual field.16
Related: 6 steps to establish an OSD advocate in your practice
The use of steroid eyedrops is often needed to treat SSDE in addition to a foundational treatment that includes one of the currently available cyclosporin offerings such as Restasis (Allergan), Klarity-C (Imprimis), Cequa (Sun), or another option such as Xiidra (lifitigrast, Novartis). I also recommend nutritional supplementation such as HydroEye (ScienceBased Health), corneal-preservation measures throughout the day like ultraviolet (UV) protection, and conscious blinking with screen use.
The use of blood-derived eyedrops like autologous serum is a suitable addition to treatment in cases involving inflammatory disease like SSDE, Stevens-Johnson’s syndrome, and ocular cicatricial pemphigoid (OCP).17-18
In SSDE, basal tear film constituents are lacking to the extent that the integrity of the ocular surface is compromised, resulting in the disruption of the ocular surface epithelium.19 The addition of autologous serum eyedrops begins to re-establish appropriate levels of biologically active tear components such as essential proteins, growth factors, vitamins, and antioxidants.17-19 Significant clinical work has demonstrated the importance of these tear components in the regulation of proliferation, differentiation, and maturation of the ocular surface epithelium.17-19
Related: How to create an ocular surface disease treatment protocol
It is important to note that the concentrations of biologically active tear components are not identical in tears and in serum.17-19 Yet, given that many of the essential components in tears are also in serum, the use of serum to supplement the tear film in OSD is feasible and serves a regenerative role which helps to restore a healthy ocular surface.17-19
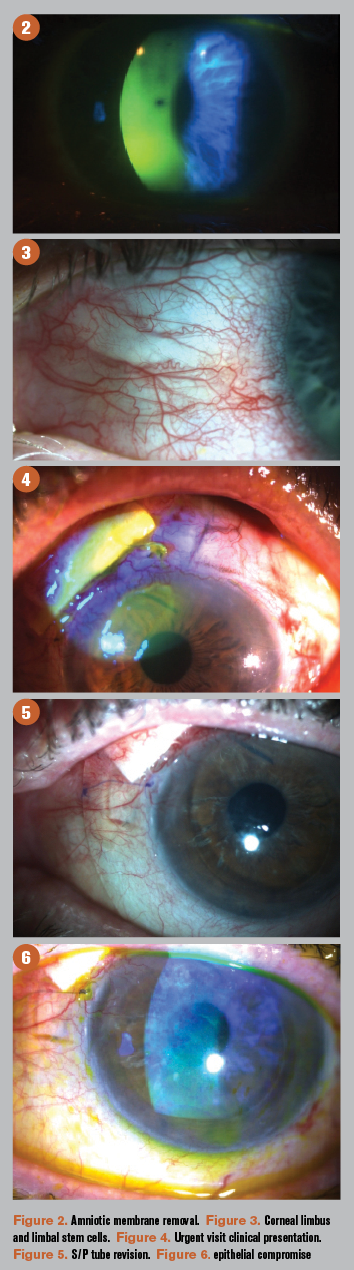
In my experience, patients with significant PEK, as in this case, also benefit from the regenerative properties of amnionic membrane (AM). After a detailed discussion with the patient, we agreed to use ProKera (BioTissue) in her left eye. On follow up, PEK improved to 1+ in each eye, along with a significant improvement in the patient’s symptoms (Figure 2).
I find that in cases with a flare-up of underlying disease or simply worsening epithelial compromise, utilizing cryopreserved AM helps to restore the corneal epithelium in 4 to 5 days.
During the patient’s next follow-up visit after the Prokera ring was removed, I recommended she give scleral contact lenses a try in an effort to continue to improve comfort. It was a good time to try contact lenses given the improved epithelium and overall inflammatory picture. To my surprise, she now feels better than she has in the last three years and is able to tolerate her scleral contact lenses 8 hours a day.
She continues to instill autologous serum eyedrops through the winter months and uses her contact lens as a reservoir by putting a drop in the contact lens each day. Scleral contact lenses can be a great therapeutic adjunct for these patients.
A key clinical landmark to assess in patients with chronic ocular surface inflammation is the corneal limbus (Figure 3). It serves as a transition zone between the avascular cornea and the vascularized conjunctiva marked by unique fibrovascular ridges known as the palisades of Vogt (POV).20-22
Related: Better manage nocturnal lagophthalmos for dry eye patients
Limbal epithelial stem cells occupying this area play a critical role in preventing conjunctivalisation and propagation of blood and lymphatic vessels into the normally avascular cornea, as well as regeneration of the corneal epithelium.20-22 Defects in limbal epithelial cells have been linked to corneal neovascularization, persistent epithelial defects, ulceration, lipid keratopathy, pain, and discomfort.20-22 Progressive limbal stem cell deficiency correlates with degeneration of the POV structures and corneal nerve deficit.20-22 The protective functions of a healthy stem cell niche have been shown to be augmented by the anti-inflammatory, anti-angiogenic, and regenerative properties of cryopreserved AM.23
Confocal microscopy imaging of the patient demonstrated abnormalities in corneal morphology. It is likely that her symptoms of intense pain-enough to wake her up in the night-are linked to corneal nerve hyperalgesia.
Related: Experts offer advice to ODs starting dry eye subspecialty
Case 1 summary
In OSD cases with significant chronic systemic inflammatory disease, consider using anti-inflammatory drops like cyclosporin and lifitegrast and nutritional supplements as a part of a foundational treatment.
Compounded topical medications like blood-derived eyedrops are an excellent adjunct for patients with epithelial compromise, aqueous deficiency, and evaporative dry eye.
Don’t lose track of macular and optic nerve assessment in patients needing steroid drops intermittently and taking HCQ or other systemic medications with a drying effect on the ocular surface.
Cryopreserved AM can offer fast epithelial recovery during a flare-up and worsening punctate epithelial keratopathy.
Young patients with significant meibomian gland dropout should be offered in-office procedures like intense pulsed light (IPL), thermal pulsation, meibomian gland probing (MGP), meibomian gland expression (MGE), and eyelid debridement every 4 to 6 months. It is important to stress to the patient that in-office treatments are to complement the use of at-home therapies like NuLids (NuSight Medical), and Zocular lid cleansing.
Confocal microscopy imaging can identify corneal nerve abnormalities in patients with chronic inflammatory disease. Epidermal growth factor (EGF) and nerve (NGF) growth factor treatment can play an important role in re-establishing a healthy interaction between the corneal nerves and the corneal epithelium.4-8,17-19
Related: S. Wade Kimmell, OD on the importance of updated Glaucoma, OSD and dry eye treatments
Case 2: Pseudoexfoliative glaucoma, corneal disease, and OSD
The patient presented for an urgent visit with ocular pain, dryness, and irriatation in the left eye.
Pertinent history OS includes:
- 20/100 best corrected Snellen visual acuity OS; has been stable for 3 years
- Severe level pseudoexfoliative glaucoma OS
- IOP target range upper teens OS; IOP 26 mm Hg on Lumigan (bimatoprost, Allergan), Combigan (brimonidine tartrate/timolol maleate, Allergan, and Azopt (brinzolamide, Novartis)
- History of Descemet’s membrane endothelial keratoplasty (DMEK) exchange for escemet’s stripping with endothelial keratoplasty (DSEK) for Fuchs
- Type 2 diabetes; no previous retinopathy
- Intolerant to oral sulfa medications
- Epithelial basement membrane dystrophy
Related: Do ODs take chronic inflammation seriously?
Surgical history OS:
- Kelman phaco-emulsification (KPE), posterior chamber lens (PCL)
- Selective laser trabeculoplasty (SLT) twice
- Trabeculectomy
- Suture lysis three times
- DSEK, IOL exchange (after IOL dislocation), vitrectomy, sutured posterior chamber lens, superficial keratectomy, temporary tarsorrhapy
- Anterior chamber washout (retained lens material)
- Bleb revision
- Ahmed Shunt with patch graft
- Repeat DSEK, tube shunt revision, superficial keratectomy, superior cauterized punctal occlusion, temporary tarsorrhapy
- Tube shunt revision
- Tube explanted
- Micropulse twice
Clinical exam OS showed:
- Complex surgical history for pseudoexfoliative glaucoma, Fuchs dystrophy, and co-morbid OSD
- Patch graft exposure through conjunctiva (Figure 4)
- Not enough healthy conjunctiva to allow complete revision; tube explanted and conjunctiva allowed to heal over patch graft (without conjunctival revision; Figure 5)
- Thin and chronically inflamed superior conjunctiva
- Diffuse PEK without filaments (Figure 6)
- Meibomian gland truncation and asymmetric tear film lipid layer deficiency
- Eyelid telangiectasia, blepharitis, and keratinization
Treatment consisted of a large-diameter contact lens until the conjunctiva healed over the patch graft. The patient was also treated with non-preserved lubricating drops, IPL, eyelid debridement/microblepharoexfoliation (MBE), MGP, and MGE. This is the foundational treatment as discussed in the previous case. The patient preferred to hold on the use of compounded medications at this point.
Case 2 discussion
I chose this case in order to demonstrate that severe OSD presents with significant comorbidity, often also requiring multiple surgical procedures. Patients requiring multiple glaucoma medications will may develop ocular surface dryness, inflammation, epitheliopathy, and decreased goblet cells.24 This challenge has spurred innovation in the delivery of drops and devices that aim to provide adequate pressure-lowering effect while avoiding ocular surface compromise.
The urgent visit presented an obvious need to cover the exposed patch graft and prophylaxis to prevent infection. Once the tube was explanted, the patient’s IOP was ultimately controlled by micropulse photocoagulation.25
Related: Your glaucoma patients also have ocular surface disease
There are many challenges to this case, including uncontrolled glaucoma, chronic OSD, and epithelial compromise after multiple surgical procedures. On follow-up with her retinal specialist, another challenge was added: the need for chronic steroid use to control cystoid macular edema (CME) (Figure 15). Despite multiple vision-threatening challenges, one of the most important things to do for this patient is re-establishing a healthy epithelium because this is directly associated with the quality of the remaining vision in the patient’s left eye. On the last visit, she exhibited some functional vision (20/100). This is remarkable, given her tough course.
In cases like this, trying to understand how to prioritize the treatment plan is critical. Once the immediate challenges of controlling the IOP, closing the corneal epithelial and conjunctival defects, and monitoring until the patch graft was no longer exposed, planning in-office treatments to improve meibomian gland function and reduce eyelid and conjunctival inflammation were an important next step.
The need for long-term Durezol as well as CME from diabetes also presents another challenge for this patient in the form of delayed epithelial healing and possible Durezol keratopathy. After offering the patient compounded blood-derived topical medications as in the first case, she was not able to keep up with the onerous drop routine and preferred to move forward with in-office procedures alone. I asked her to start compounded Healon (1.4% sodium hyaluronate, Johonson & Johnson Vision) qid and will continue to offer autologous serum or similar treatments with regenerative properties to the corneal epithelium.
Related: Remember the basics as dry eye treatments expand
I find the combination of MBE, IPL, MGP, MGE alternating with MBE, TearCare thermal pulsation (Sight Sciences), and MGE every 4to 6 months very useful in these situations. I prefer to use TearCare for thermal pulsation on all patients with history of a trabeculectomy in order to preserve the conjunctiva as much as possible. This is an important consideration given this patient’s conjunctival surgical history. Eyelid keratinization and telangiectasia are treated with MBE and IPL. MBE is an important step that can often be missed in these cases.
Taking the time to debride biofilm and keratinized epithelium will aid the provider in allowing more secretion from the meibomian glands to the ocular surface. Maintaining this reduction in biofilm with daily treatment at home is key.
Case 2 summary
When multiple comorbidities present along with severe OSD, remember the main things. For example, IOP control and conjunctival and corneal epithelial healing with prophylaxis against possible infection on her urgent visit.
Stabilize the patient with acute problems while formulating a long-term plan to maximize the best remaining visual function. Despite complex history and severe glaucoma, and now CME (which did not require injection), the patient still has functional vision in this left eye. It is worth fighting to preserve.
Understand the main, ongoing challenges to epithelial compromise and OSD. In this case, the need for multiple pressure-lowering drops, complex surgical history including failed bleb, tube explant, repeat corneal transplant, need for Durezol to control CME, delayed healing with diabetes, meibomian gland disease, and blepharitis.
Related: Why OAB should be considered before cataract removal
Conclusion
ODs are now able to provide a higher-level of care to patients with significant ocular surface disease and comorbid ocular and systemic disease. With increased awareness of OSD and more attention to this unmet need, patient outcomes are improving.
Paring OSD treatment options with each patient is a skill that continues to evolve as ODs learn where to best utilize new technology. Mutually arriving at the right treatment path with the patient after articulating the risks, benefits, alternatives, and limitations of each recommendation is a complex process when faced with complex disease, yet one of the most rewarding things we can offer patients with significant disease.
References:
1. Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017 Jul;15(3):575-628.
2. Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004 Mar;78(3):409-16.
3. McKown RL, Wang N, Raab RW, Karnati R, Zhang Y, Williams PB, Laurie GW. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009; 88(5):848-858.
4. Bonini S, Lambiase A, Rama P, Filatori I, Allegretti M, Chao W, Mantelli F; REPARO Study Group. Phase I Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018 Sep;125(9):1468-1471.