FDA grants 501(k) clearance to CorNeat Vision’s EverPatch
EverPatch is described by the company as the world's first non-degradable, synthetic tissue substitute for ophthalmic surgery.
According to the company, the CorNeat EverPatch is “poised to displace the use of donor and processed tissue, commonly utilized in ocular surgeries but posing the risk of disease transmission.” (Adobe Stock / Tada Images)

CorNeat Vision announced the US Food & Drug Administration (FDA) has granted 501(k) clearance to the company’s EverPatch.
In a press release from CorNeat Vision,1 the CorNeat EverPatch is described as “the first synthetic, non-degradable tissue-integrating matrix for use in ophthalmic surgeries,” and is composed “a non-woven, polymer matrix which integrates with surrounding tissue and is intended to reinforce the sclera and aid the physical reconstruction of the ocular surface.”
According to the company, the CorNeat EverPatch is “poised to displace the use of donor and processed tissue, commonly utilized in ocular surgeries but posing the risk of disease transmission.”
Gilad Litvin, MD, CorNeat Vision’s Chief Medical Officer and Co-Founder discussed the goals of the CorNeat EverPatch in the press release from the company.
"The ideal graft material should be long-lasting, sterile, immunologically inactive, cosmetically acceptable, and readily available. The CorNeat EverPatch was designed with these goals in mind,” said Litvin., “Our novel ophthalmic patch is significantly thinner than processed patch tissue, provides better handling as it does not 'cheesewire' when sutured, and has holes that allow for accurate positioning and anchoring. These holes also facilitate direct conjunctival adhesion to the sclera thus supporting its bio-integration.”
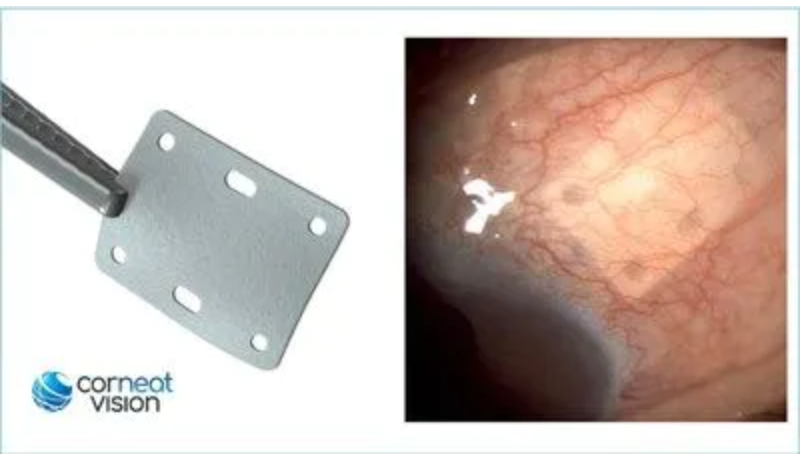
Left - CorNeat EverPatch. Right - CorNeat EverPatch implanted under the conjunctiva, 9 months post implantation. (Photo Courtesy CorNeat Vision)
Almog Aley-Raz, CorNeat Vision’s CEO and VP R&D, talked about the technology behind the CorNeat EyePatch in the release.
“It is the first device that leverages the EverMatrix™, our core tissue-integrating material platform technology, originally developed for our corneal prosthesis program,” said Aley-Raz. “EverMatrix™ presents a significant business opportunity as it is the only synthetic non-degradable patch material in ocular surgery. This biocompatible material has the potential for wider use in soft tissue reinforcement, biomechanical integration of implants with surrounding tissue, fabrication of membranes, and concealment of implants and sensors.
The company states the CorNeat EverPatch will be launched initially in leading ophthalmic centers in the US in Q3 2023, expanding nationwide later in the year.
References:
1. FDA Clears CorNeat EverPatch, World's First Non-degradable, Synthetic Tissue Substitute for Ophthalmic Surgery. Press Release. CorNeat Vision; June 12, 2023. Accessed June 12, 2023. https://www.prnewswire.com/news-releases/fda-clears-corneat-everpatch-worlds-first-non-degradable-synthetic-tissue-substitute-for-ophthalmic-surgery-301847803.html
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.