Tonometer offers reliable way to measure intraocular pressure
A handheld tonometer that measures IOP through the eyelid and over the sclera is proving helpful for optometrists faced with patients who are apprehensive about seeing an instrument approaching their eyes.
Long Beach, NY-A handheld tonometer (Diaton, BiCOM Inc.) that measures IOP through the eyelid and over the sclera is proving helpful for optometrists faced with patients who are apprehensive about seeing an instrument approaching their eyes or who have corneal abnormalities.
The novel device is a pen-like instrument that measures IOP within seconds without the need for anesthesia or sterilization. Approved by the FDA in 2006, the instrument has been the subject of numerous clinical trials, where it has been found comparable to the eye doctors' "gold standard" (Goldmann applanation tonometer, Haag-Streit).
According to Roman Iospa, chief executive officer of BiCOM, the device is available in more than 50 countries, and more than 5,000 units are on the market.
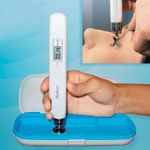
"It is convenient for the doctor and painless for the patient," Iospa said. "There is really no discomfort, especially for the patient who might be slightly anxious."
H. Arnold Papernick, OD, an optometrist in private practice in Mt. Pleasant, PA, said the device has been helpful when examining pediatric patients or anyone who is averse to objects coming at the eye. Excessive blinking can cause IOP measurements to read abnormally high, he explained.
"I had (a patient) who was getting high readings (because of blinking)," Dr. Papernick said. "Then I did it with the (novel tonometer) and I got some beautiful results (that correlated with earlier readings by an ophthalmologist)."
Because patients are told to recline slightly and look up for the readings, Dr. Papernick found it helpful to put an inverted cup on the ceiling as a focal point. He said the novel tonometer is easier for children because it takes measurements quickly.
Comparison testing
According to several clinical trials, the device offers results that are comparable to the "gold standard" tonometer. Richard S. Davidson, MD, in private practice at the Rocky Mountain Lions Eye Institute, Aurora, CO, led a retrospective chart review of consecutive IOP measurements performed on 64 eyes of 32 patients aged 34 to 91 years with both the novel tonometer and "gold standard" tonometer. He found that 83% of all measurements were within 2 mm Hg of each other.
"The transpalpebral method of measuring IOP with the (novel tonometer) correlates well with (the "gold standard" tonometer)," the study concluded. "(The novel tonometer) may be a clinically useful device for measuring IOP in routine eye exams."
A similar retrospective review, led by Theodore H. Curtis, MD, at the Rocky Mountains Lions Eye Institute, found that the novel tonometer's measurements correlated well with the hand-held instrument's (Tono-Pen, Reichert Inc.) measurements. The study noted that the novel tonometer was useful when examining children, who "were reassured by the fact that no drops were needed."
Mark Latina, MD, and Tarek Shazly, MD, members of the department of ophthalmology, Massachusetts Eye and Ear Infirmary, Boston, and Emil William Chynn, MD, an ophthalmologist in private practice at Park Avenue Laser, New York, performed a study of the novel tonometer to compare its IOP measurements with those from the "gold standard" tonometer in normal and glaucomatous eyes.
The study examined 66 eyes of 33 consecutive subjects, 46 eyes having glaucoma and 20 eyes without. The "gold standard" tonometry was performed by one of the authors, while the novel tonometer measurements were taken by another author in a masked fashion. In both the normal and glaucoma groups, 15.15% of the novel tonometer's measurements were exactly the same as those obtained with the "gold standard" tonometer. The novel tonometer underestimated the IOP compared with the "gold standard" tonometer in 37.87% of eyes, and over-estimated the IOP in 43.93% of eyes.
However, the novel tonometer correlated within 3 mm Hg of the "gold standard" tonometer in 83.3% of eyes, and "may be a clinically useful screening device for measuring IOP," the authors concluded.
"At the end of the study, we reached the conclusion that the transpalpebral technique is a very promising method for tonometry, especially for screening and in patients with corneal pathology," Dr. Shazly said.
Dr. Chynn said the device might be a good instrument for family practitioners for glaucoma screening because it does not require anesthesia and can be performed simply in an office setting.
"The (novel tonometer) is easy to use, user-friendly, and can be used on patients for mass screening," Dr. Shazly added.
Meanwhile, John Hope, MD, an ophthalmologist in private practice in Oklahoma City, OK, said he is impressed with the instrument and prefers it because patients "hate the non-contact tonometry" and applanation tonometry is time-consuming and often requires support staff. He has used the instrument routinely on every patient for at least 6 months.
Dr. Hope said, "There are no rubber covers to deal with, and, after the initial purchase, it is virtually maintenance-free. It is easily portable in your pocket and can be transported from room to room or office to office.
"There is no corneal contact and pressures can be obtained in patients wearing contact lenses. The technique is easily and quickly learned, and the company has excellent training instructions," he concluded.
Newsletter
Want more insights like this? Subscribe to Optometry Times and get clinical pearls and practice tips delivered straight to your inbox.