- Therapeutic Cataract & Refractive
- Lens Technology
- Glasses
- Ptosis
- AMD
- COVID-19
- DME
- Ocular Surface Disease
- Optic Relief
- Geographic Atrophy
- Cornea
- Conjunctivitis
- LASIK
- Myopia
- Presbyopia
- Allergy
- Nutrition
- Pediatrics
- Retina
- Cataract
- Contact Lenses
- Lid and Lash
- Dry Eye
- Glaucoma
- Refractive Surgery
- Comanagement
- Blepharitis
- OCT
- Patient Care
- Diabetic Eye Disease
- Technology
MIGS dramatically transforms glaucoma management
Revolutionized treatment offers favorable safety profile, quick postop recovery
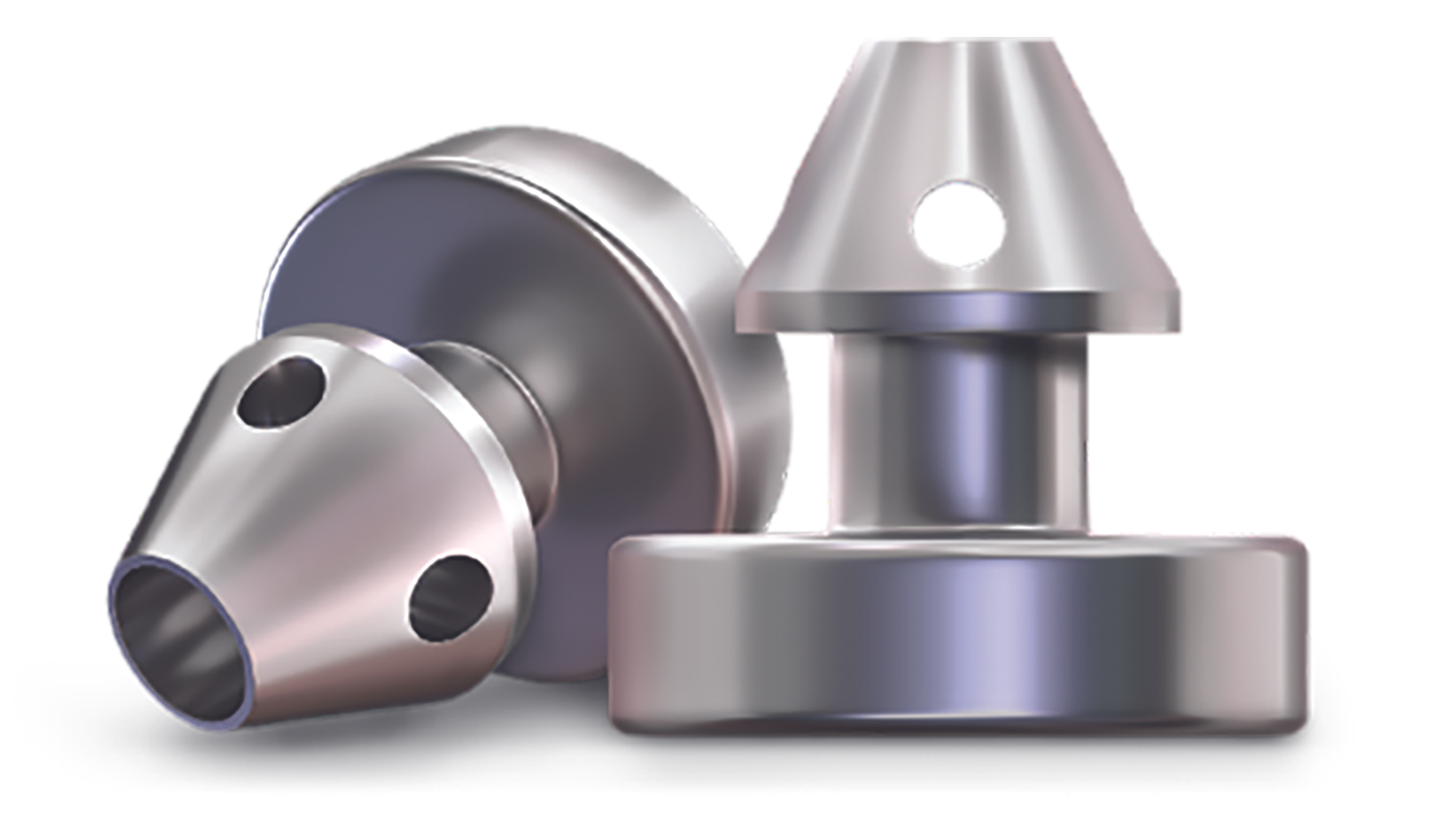
Figure 1.The iStent inject W by Glaukos.

Although diagnosing glaucoma has advanced modestly in recent years, the management of the disease has undergone a true transformation. In particular, management has shifted toward intervention that is earlier, safer, and often more surgical.
In the first installment of our 2-part series on glaucoma treatment, we looked at the introduction of new medications and the role of lasers. In this follow up exploration within the glaucoma treatment landscape, we will focus on minimally invasive glaucoma surgeries (MIGS).
MIGS
Glaucoma management has been revolutionized by the introduction of MIGS, a heterogeneous group of procedures that achieve reliable IOP reduction with a favorable safety profile and quick postoperative recovery. MIGS are becoming increasingly popular and are already an integral part of the glaucoma management tool kit.1 Although the most obvious benefit of MIGS is the lowering of IOP, other potential advantages include reduction of medication load, preservation of the ocular surface, and the decrease of diurnal IOP fluctuation.2 Decreasing the frequency of eye drops can be very important to patients susceptible to ocular surface disease.
Because MIGS offer good IOP reduction and low adverse effect (AE) profiles, ophthalmologists are using them more frequently than the traditional incisional surgeries and earlier in the course of the disease. The earlier a MIGS procedure is performed, the more lifetime benefit accrues for the patient.
There is a significant shortage of glaucoma specialists in the United States, so MIGS procedures increasingly are performed by general ophthalmologists and cataract surgeons. There may even arise a niche category of ophthalmologists specializing in MIGS. Optometrists should identify local surgeons who perform MIGS procedures and develop a working relationship with them. Knowing which procedures a local surgeon generally performs helps in setting patient expectations prior to a referral. MIGS fit well into various comanagement models and the postoperative care is often like that of cataract surgery.
MIGS that remove part of the angle anatomy
Most MIGS procedures work by increasing aqueous outflow, although some reduce aqueous production. Many MIGS can be used in combination with cataract surgery but an increasing number have been approved for standalone use.
Historically, the trabecular meshwork (TM) has been considered the primary site of outflow resistance, which is why the majority of MIGS procedures aim to remove or bypass the TM. Gonioscopy-assisted transluminal trabeculotomy (GATT) and the Kahook Dual Blade (KDB; New World Medical) are 2 ways to remove large portions of the TM.
During the GATT procedure, the surgeon passes a Prolene suture all the way around through Schlemm’s canal and then pulls on both ends to tear open the roof of the canal 360°. Retrospective studies have shown a 37% to 49% IOP reduction in patients with various types of glaucoma, with IOP frequently settling in the midteens after the procedure.3,4 Prospective studies, however, are lacking. Regarding postoperative complications, hyphema is common in the first week after GATT but is usually self-limiting.
The KDB is a purpose-built blade with a footplate that settles easily into the posterior bed of the Schlemm canal. The surgeon then slides the device around 90° of canal as 2 parallel blades excise a clean strip of the canal roof. Current small studies show that the IOP-lowering effect of KDB is rather modest.5,6 Larger prospective studies are needed.
Figure 2.The iStent inject by Glaukos.
MIGS that bypass the TM
TM resistance also can be bypassed with a stent, such as the iStent inject (Glaukos), which is the smallest implantable device on the market.7 The current iteration of this device, the iStent inject W (see Figure 1 and 2), involves injecting 2 small stents through the TM. The stents provide a pathway to bypass the TM resistance. The devices are usually inserted nasally, where most collector channel entrances are located. This procedure is currently only approved in conjunction with cataract surgery for patients with mild-stage or moderate-stage glaucoma.8 There is significant evidence that the combination of iStent inject and cataract surgery lowers IOP more than cataract surgery alone.9,10 On the other hand, the injection mechanism and the small size of the stents make it more difficult to ensure that the devices are in close vicinity of a collector channel entrance. This may explain some inconsistencies with this stent’s performance.
Figure 3.The Hydrus Microstent (Ivantis), pictured for size comparison, is a curved and fenestrated implant that inserts into the canal. (All images courtesy of Gleb Sukhovolskiy, OD)

MIGS that open Schlemm’s canal
Recent studies have suggested that the TM might not be the main site of aqueous resistance in primary open-angle glaucoma (POAG) but rather that the IOP elevation is caused by a combination of 3 factors: loss of permeability of the TM, collapse of Schlemm’s canal, and downstream resistance with the closing of collector channel entrances.11-13 It is now apparent that Schlemm’s canal plays a significant role in facilitating aqueous outflow, because dilation of the canal is directly correlated with the magnitude of IOP reduction.14,15
One way to open Schlemm’s canal and improve access to the collector channel entrances is by performing a canaloplasty, a mechanical dilation of the canal. This is typically performed by distending the canal using a pressurized injection of viscoelastic; the viscoelastic is then removed, leaving no tissue damage and no devices in the eye, only a reformed canal. Although canaloplasty has been utilized for some time, it has undergone a shift in the age of MIGS, becoming safer and easier to perform. Much of this has involved a shift from the older ab externo approach (in which the surgeon dissected down to Schlemm’s canal through conjunctiva and sclera) to the ab interno approach (in which the surgeon approaches the canal from a corneal incision and the anterior chamber).16
There are currently 2 devices that perform viscocanaloplasty: the OMNI Surgical System (Sight Sciences Inc) and the Ab-interno Canaloplasty using the iTrack Surgical System catheter (Ellex iScience).17,18 These devices are quite similar, although the OMNI can also perform a trabeculotomy in addition to the canaloplasty.
One of the benefits of viscocanaloplasty is that it targets all 3 areas of aqueous resistance without any physical device being implanted in the eye.
Both surgeries may be used as standalone procedures or together with cataract surgery, and in any stage of glaucoma. Early results are promising, but long-term studies are needed to determine lasting effect.19,20
Viscocanaloplasty together with trabeculotomy may be a good option for a pseudophakic patient with glaucoma for whom a moderate reduction in IOP is needed but incisional glaucoma surgery seems too risky.
Although mainly considered to be a TM bypass device, the Hydrus Microstent (Ivantis) also addresses Schlemm’s canal collapse. The Hydrus (see Figure 3) is a curved and fenestrated implant that inserts into the canal, essentially scaffolding it from the inside. Although the Hydrus only occupies 90° of Schlemm’s canal, it is usually inserted inferonasally, the area with the highest concentration of collector channel entrances. The Hydrus Microstent has strong data supporting its efficacy.
The pivotal HORIZON trial (NCT01539239) demonstrated consistent results for the Hydrus Microstent over a 5-year follow-up period, a longer follow-up period than studies of any other MIGS devices.21
The most notable findings include consistent IOP control and decreased risk of future incisional surgery compared with cataract surgery alone. Though not available as a standalone procedure in the United States, Hydrus fared better than 2 iStents in a large comparison study.22 Hydrus should be strongly considered for patients with POAG undergoing cataract surgery.
MIGS that reduce aqueous production
Endoscopic diode cyclophotocoagulation (ECP) is the only MIGS procedure that reduces aqueous production. It does this by using a laser to selectively target and damage the ciliary body. For most of its history, cyclophotocoagulation had a high risk of AEs including hypotony, inflammation, vision loss, and phthisis bulbi, so it was mostly used in refractory glaucoma to reduce IOP in blind and painful eyes and could hardly be considered minimally invasive.23
Recent improvements, including less energy use and more targeted treatment, have helped decrease the rate of severe complications. With these improvements, surgeons are more likely to consider ECP for milder forms of glaucoma. However, ECP is still far from achieving mainstream acceptance as a MIGS procedure because of the low but nonnegligible risk of permanent vision loss.24-26
MIGS that shunt aqueous to the subconjunctival space
As the only MIGS device to completely bypass the conventional outflow pathway, the Xen Gel Stent (Allergan) straddles the border of MIGS and incisional glaucoma surgery. It is a tiny gelatin stent that shunts fluid from the anterior chamber into the subconjunctival space, bypassing the trabecular outflow pathway completely.
This action is similar to how glaucoma tube shunts work, but because the procedure can be performed through a small corneal incision (the ab interno approach) the Xen Gel belongs in the MIGS category.
The FDA has approved Xen Gel as a standalone procedure or in conjunction with cataract surgery. The stent’s effect appears to be quite robust, with an average drop in IOP of 8.45 mm Hg seen in 1 study.27
According to results of another study, at 2 years patients with the stent had an average 29.3% reduction in IOP.28 Although not without risk, its safety profile is better than that of either trabeculectomy or tube shunts.29,30 Patients typically discontinue all glaucoma medications immediately after the surgery.
Unlike the angle-based MIGS procedures, in which postoperative care is like that of cataract surgery, postoperative care after the Xen Gel stent is more involved and heavy steroid use is important in preventing bleb scarring. Despite this, Xen Gel recipients require a bleb needling more than 40% of the time.31
Incisional surgery
When laser therapy, medications, and MIGS are not effective in controlling IOP, incisional surgeries such as trabeculectomy or glaucoma drainage device implantation can be performed to create a new pathway for the egress of aqueous humor.
Trabeculectomy has been the gold standard of glaucoma surgery for decades, but its use has declined steadily due to improvements in medication efficacy and the proliferation of less-invasive surgical options.32,33 Trabeculectomy has high rates of complications including hypotony, choroidal effusions, hyphema, bleb leakage, blebitis, and endophthalmitis. Although it can achieve excellent IOP control in the short term, trabeculectomy has a high failure rate at 5 years.34
On the other hand, glaucoma drainage implants (GDIs) have increased in use, in large part due to results of the Tube Versus Trabeculectomy study (NCT00306852), which showed a lower rate of failure for GDIs compared with trabeculectomy.35
The 2 most-used drainage implants are the Ahmed Glaucoma Valve (New World Medical) and the Baerveldt Glaucoma Implant (Johnson & Johnson Vision). The Baerveldt implant reduces IOP more significantly over the long term than the Ahmed but carries a higher risk of hypotony and other complications. Conversely, the Ahmed implant reduces the rate of complications but is less likely to achieve the desired IOP reduction.36,37
The decision regarding whether to perform a trabeculectomy or to use a GDI depends on each surgeon’s familiarity and comfort with these surgeries and the goals the surgeon is hoping to achieve.
A new paradigm
We are in the midst of a paradigm shift in the management of glaucoma. Our management of this disease has shifted toward intervention that is earlier, safer, and often more surgical. We now know that a large portion of patients do not use their eye drops as prescribed, and that MIGS can produce more consistent results.
The success and safety of MIGS have encouraged many glaucoma specialists to speculate that we are entering a period in which lower risks and improved IOP-lowering abilities are turning glaucoma into a primarily surgical disease.
There is a growing consensus that early-stage glaucoma is often the best time to intervene. As the safety profile of MIGS improves, providers are no longer waiting until a patient is on 3 or 4 glaucoma medications before considering surgery.
The field of glaucoma has become significantly more exciting and is evolving quickly. The concepts upon which we once relied are being questioned, and new ones regularly challenge our way of thinking. The more familiar we become with the new tools, the sooner we can decrease the vision lost to this disease.

