
Key clinical trial data and PDUFA dates in early 2026 focus on treatments for dry eye, age-related macular degeneration, and rare eye diseases.

Key clinical trial data and PDUFA dates in early 2026 focus on treatments for dry eye, age-related macular degeneration, and rare eye diseases.

A total of 38 patients were included in the study. All underwent implantation of the PRIMAretinal prosthetic chip with the goal of restoring vision.

Employers are obliged to provide employees with appropriate safety eyewear and face protection that conforms to the industry standard.

“Remarkable” results were reportedly obtained with hypotony with hydroxypropyl methylcellulose.

Investigators presented genomic, transcriptomic, histological and functional evidence that the Greenland shark retains an intact visual system well-adapted for life in dim light.

Evolutions in AI, myopia management, and individualized care are on ODs' minds for the new year.

The FDA issued a CRL for ONS-5010, citing the need for additional confirmatory efficacy evidence despite previous trials showing efficacy, according to the company.

A recent study represents a step forward in investigations of the retinal layers as they are affected by glaucoma.

New drugs on the market and survey findings were among some of the top stories in dry eye from the past year.

Justin Schweitzer, OD, outlines the intricacies of glaucoma care with 3 cases.

Optometrists have their eyes set on ocular surface disease treatment and contact lens technology advancement.

The grading scales were compiled with the goals of achieving conciseness and the ability to provide clear drug-dose-modification recommendations compared with the previous ocular CTCAE scale.

An effective but underused resource with positive benefits for patients with myopia.

ODs are in a prime position to identify exploited victims.

Maturing of the vestibulo-ocular reflex is independent of sensory input.

New technology in all facets of eye care provides an opportunity for eye care providers to step out of their comfort zones.
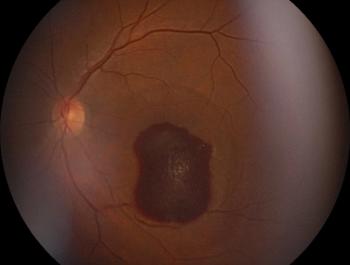
In the study, authors investigated the natural history of GA lesion incidence rates and analyzed potential risk factors for faster incidence of GA lesions.

This retrospective cohort study used a large US administrative claims database to identify patients with a diagnosis of seborrheic dermatitis from January 1, 2016, through June 30, 2022.

Clark Chang, OD, MSA, MSc, FAAO; and Michelle Chung, OD, FAAO, FSLS, provided cornea updates during the American Academy of Optometry Academy 2025 conference.

Johnson & Johnson and The Contact Lens Institute were among industry leaders that provided research and study results during the conference.

Dry eye disease is ubiquitous, with up to 20% of middle-aged patients estimated to have moderate to severe symptoms or searching for a treatment.

Raman Bhakhri, OD, FAAO; Julie Rodman, OD, MS, FAAO; and Andrew Rixon, OD, provide retinal updates during the meeting.
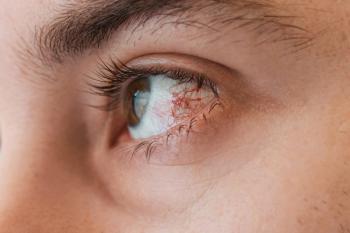
NK is more common than recognized; early diagnosis is paramount.

Optometrists and industry leaders alike share exciting new developments in myopia management.

Fovea-sparing, multifocal, and bilateral lesions exhibited the fastest growth rates.

Monica Jong, PhD, BOptom; and Ryan Parker, OD, spoke on the importance of the lens at this year's Academy meeting.

The National Eye Institute Visual Function Questionnaire was a tool that has been used previously to assess the patient-reported vision-related quality of life in patients with floaters.

Eye care providers are uniquely positioned to catch early risks during routine exams.

Keeping an eye on CE, industry news, and collaboration in Boston, Massachusetts.
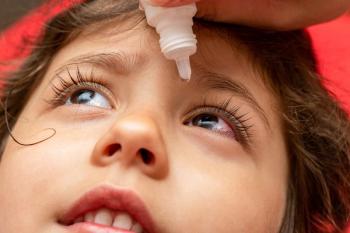
The researchers noted the importance of comprehensive eye examinations, including cycloplegic refraction for diagnosing and managing common pediatric ocular conditions, especially in children who fail vision screening.

Published: November 5th 2025 | Updated:
Published: September 6th 2023 | Updated:

Published: December 31st 2025 | Updated:

Published: September 19th 2025 | Updated:

Published: November 4th 2021 | Updated:
Published: March 10th 2022 | Updated: