
Study participants were followed annually from 2006 to 2018 with cycloplegic refractions and ocular biometry; the parents were followed with noncycloplegic refractions and ocular biometry.

Study participants were followed annually from 2006 to 2018 with cycloplegic refractions and ocular biometry; the parents were followed with noncycloplegic refractions and ocular biometry.

Repetitive head impacts (RHIs), defined as blows to the head that do not elicit clinical signs or symptoms of concussion, occur more frequently in athletes who participate in contact sports.

IPV is defined as physical violence, sexual violence, stalking, or psychological aggression that is committed by a current or former spouse or dating partners.

The beauty of this conference is its collaborative and comprehensive nature.

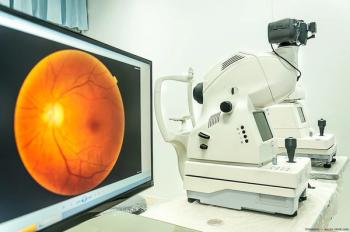
Researchers conducted this study to determine how often and why spontaneous soft drusen regresses without atrophy in patients who had intermediate or atrophic age-related macular degeneration.

Research reveals that increased exposure to green spaces significantly reduces the risk of developing dry eye disease, highlighting urban planning's vital role in public health.


Increased amounts of time spent in physical activity are independent predictors of a slower rate of visual field mean deviation loss in patients with primary open angle glaucoma.

Boston event will shed light on cerebral/cortical visual impairment (CVI), a leading cause of pediatric blindness

A rare case of tubulointerstitial nephritis and uveitis syndrome in a teenager highlights the importance of multidisciplinary diagnosis and management.
Ocular salvage and preservation of vision are essentially dependent on early diagnosis, and several studies have been conducted to identify the factors leading to delayed diagnosis at presentation.

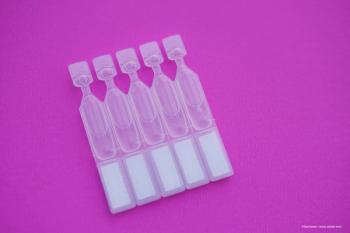
The tool is designed to help with the application of mono-dose, dry eye eye drop vials more easily and accurately.

New research reveals collagen mimetic peptides may effectively restore ocular tissue stiffness, offering hope for myopia and other eye diseases.

Ophthalmologists report high satisfaction with lifitegrast for dry eye treatment, noting rapid symptom relief and effective management in diverse patient populations.

A recent study reveals that ADHD in schoolchildren correlates with lower myopia prevalence and shorter eye length, suggesting intriguing links between vision and attention disorders.

The primary study outcomes were the cumulative incidence rate of myopia, with myopia defined as an SER −0.50 diopter (D) or less and the percentage of participants who had a fast myopic shift that was defined as a spherical equivalent myopic shift of 0.50 D or greater over the course of 1 year.

A study reveals that secondhand smoke significantly increases the risk of myopia in Chinese children, highlighting the need for smoke-free environments.

New clinical trials reveal PER-001 as a groundbreaking treatment for glaucoma and diabetic retinopathy, showing significant vision improvements and safety.

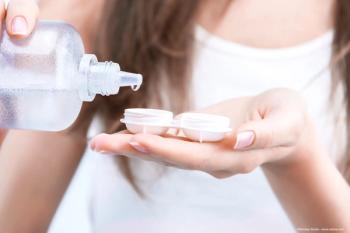
Contact lens users report high satisfaction with Biotrue Hydration Plus Solution, highlighting comfort and effectiveness during extensive wear and digital device use.

Lifitegrast 5.0% significantly improves clinical signs and biomarkers in dry eye disease, demonstrating effective results within 12 weeks of treatment.

Andrew E. Chan, OD, and Daisy Chan, OD, FAAO, discussed microplastic toxicity in the eye at Optometry's Meeting in Minneapolis, and focused on risks associated with long-term contact lens wear and topical therapies.
ST-100 shows promise as a fast-acting treatment for dry eye disease, offering rapid relief and unique collagen repair mechanisms.

A $2.5 million endowment establishes the Herbert Simon Chair in Glaucoma Research and Innovation, advancing treatments and research led by renowned glaucoma researcher Alon Harris, PhD, MS, FARVO.

A study reveals a genetic link between instant coffee consumption and increased risk of dry age-related macular degeneration, urging caution for high-risk individuals.

The study was an outgrowth of the phase 1 and 2 Ocular Hypertension Treatment Study (OHTS).

Increases in the doses of atropine were most common following the pandemic and especially among patients for whom therapy was initiated with the 0.01% dose.