Heidelberg Engineering announces FDA clearance for ANTERION diagnostic platform
The ‘all-in-one’ platform combines specialized imaging and three new FDA-approved apps with a focus on metrics, corneal imaging, and cataract care.
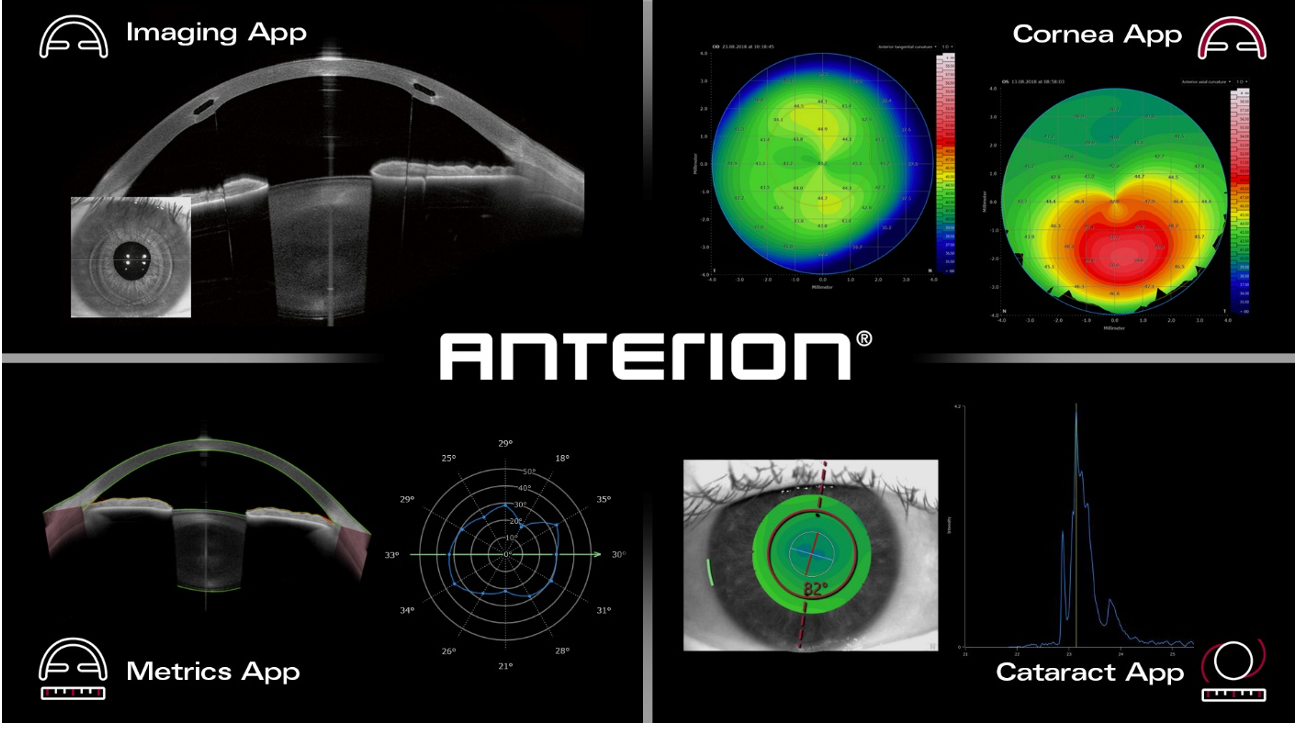
The ANTERION will work in conjunction with FDA-approved apps for imaging, metrics, cornea and cataract care. Image courtesy of Heidelberg Engineering GmbH.
Heidelberg Engineering announced today that it received clearance from the FDA for its ANTERION imaging and workflow platform. In Europe, the ANTERION already received a CE mark; the FDA clearance will expand availability to the United States.
According to a press release from Heidelberg Engineering, the ANTERION combines proprietary features with best-in-class diagnostic tools, such as IOL power calculation with corneal topography and tomography, high-resolution imaging and anterior chamber metrics. Additionally, the ANTERION features “patented eye tracking and composite imaging technologies” unique to Heidelberg.1
"By developing an all-in-one solution, it is now possible for eye doctors and technicians to perform the most important anterior segment examinations on a single device," Ram Liebenthal, US general manager of Heidelberg Engineering, Inc., exclusively told Optometry Times. "Eliminating the need to move the patient from machine to machine offers an unexpected convenience." He explained that the all-in-one ANTERION benefits clinicians and patients by making visits more efficient and minimizing the device's footprint, an especially crucial need for practitioners in small spaces.
In 2021, the FDA cleared Heidelberg Engineering’s Imaging App for anterior segment visualization. The ANTERION system will work with the Imaging App, and a suite of new apps recently cleared by the FDA. Those new additions include the Cataract App (including integrated monofocal and toric IOL calculators), the Cornea App (including corneal geometry measurements), and a Metrics App (including swept-source OCT images, a radial view of the anterior chamber, and free-hand measurements).
"We are proud that we have been able to bring the legacy of Heidelberg image quality to the front of the eye because we believe that high-resolution imaging can help remove unwanted guesswork, support clinical decision-making, and avoid refractive surprises. Thinking about the old saying, a picture is worth a thousand words, we hope that ANTERION’s stunning image quality will help patients better understand their eye condition and increase compliance in their treatment plan," Liebenthal added.
Arianna Schoess Vargas, managing director of Heidelberg Engineering GmbH, said the company is “thrilled” to make the ANTERION available to providers in the US. “We believe this device complements our current portfolio of products and arms anterior segment focused practices with the comprehensive data needed to support diagnostic decisions that ultimately improve patient care,” she said in the news release.
Mitchell W. Dul, OD, MS, principal investigator of the ANTERION clinical trial, spoke about the capabilities of the imaging device. "We were impressed by the high-quality images and measurements of the anterior segment that ANTERION offers,” he said. “Its multi-faceted utility, high resolution, rapid image acquisition, and intuitive user interface will make this device an invaluable tool for clinical practice."