
A team of Chinese researchers have been working on a way to continuously and more comfortably detect these tiny fluctuations in pressure, such as contact lenses that transmit signals to receptor glasses.

A team of Chinese researchers have been working on a way to continuously and more comfortably detect these tiny fluctuations in pressure, such as contact lenses that transmit signals to receptor glasses.

The 2 FDA registration trials for perfluorohexyloctane resulted in scientifically significant results for improvement of mild and moderate symptoms of dry eye disease.

Neda Gioia, OD, CNS, FOWNS, CFMP, details recent findings on the efficacy of the NutriTears supplement on dry eye symptoms.
Inder Paul Singh, MD, defines what it means to have an interventional mindset when it comes to glaucoma procedures and treatments during the Controversies in Modern Eye Care 2024 meeting.

Kelsey Roelofs, MD, gives her best methods to identifying and diagnosing thyroid eye disease in her Controversies in Modern Eye Care 2024 presentation.

Marc Bloomenstein, OD, FAAO, discusses how optometrists can proactively manage Demodex blepharitis by regularly examining eyelids, diagnosing the condition early, and offering treatments to effectively eradicate the mites and prevent future complications.

Arjan Hura, MD, gives an overview on a captivating retina panel and other enticing items at the CIME 2024 meeting.

James A Katz, MD, details treatment options for Demodex blepharitis, from tea tree oil to XDEMVY.

The Patient Whisperers Cofounders Lauren Weaver and Christine Scarlett gave their keynote presentation "The Secret to Crazy Growth: Defining Your Identity on Social Media," at the 2024 Controversies in Modern Eye Care meeting May 4 in Los Angeles, California.

The WVU Health System Board of Directors recently approved $233.5 million to fund the construction of a multi-center outpatient facility with additional exam and testing rooms.

OCU410 utilizes an AAV delivery platform for the retinal delivery of the RORA gene, the company stated in a news release.

The goal of the grant funding from the Choroideremia Research Foundation is to support validation of functional vision assessments for patients with profound blindness.

The company stated that 46.2% of patients demonstrated a 1- or 2-step improvement in the Diabetic Retinopathy Severity Scale (DRSS) at 40 weeks in the Axpaxli arm, compared to 0% in the control arm.

The Phase 3 study will have a sample size of 150 participants—one arm of 75 participants with the RHO gene mutation and the other arm with 75 participants that are gene agnostic.

The effort led to the creation of an artificial vitreous body for treating retinal detachment. This solution is based on a natural carbohydrate derived from algae.

Phentolamine ophthalmic solution 0.75% is a preservative-free, stable eye drop, which blocks the α1 receptor within the iris dilator muscle without affecting the ciliary muscle.

Since its U.S. launch in September 2023, more than 40,000 avacincaptad pegol intravitreal solution vials have been distributed to physician practices.

According to the company, its Virtual Eye Pro features improve visual field testing for practice and patients.

The ANX007 global pivotal program is the first to use vision preservation as a primary outcome measure in GA.

According to the company, AGTC-501 was generally safe and well-tolerated and showed improvements in visual function at the 12-month analysis. The Phase 2/3 VISTA trial for AGTC-501 in XLRP expected to begin in in the first half of 2024.
In a news release, the FDA noted these are copycat eye drop products that consumers can easily mistake for Bausch + Lomb’s Lumify brand eye drops, an over-the-counter product approved for redness relief.
The new contact lenses contain micro-sensors which monitor changes in IOP over a period of several hours, sending the data collected wirelessly so it can be analyzed by an eye care provider and a diagnosis given.
According to researchers, the strongest biomarker was achieved from a single bright flash of light to the right eye, with AI processing significantly reducing the test time.

Available information includes a new promotional toolkit for The Glaucoma Community, glaucoma patient testimonial, and medical expert interview videos.

According to Ocuphire Pharma, phase 3 startup activities are underway, and the clinical trial is expected to start in the first quarter of 2024.

Vezocolmitide is the first drug candidate based on PolyCol, Stuart Therapeutics' patented synthesized polypeptide collagen mimetic peptide platform.

iDose TR, a micro-invasive intraocular implant designed to lower IOP in patients with open-angle glaucoma or ocular hypertension, has received FDA approval following a new drug application (NDA) submission.

OCU410 is a modifier gene therapy product candidate being developed by Ocugen for dry AMD.

The company announced key secondary endpoints were achieved with both EYP-1901 doses. These include a more than 80% reduction in treatment burden, with nearly two-thirds of eyes supplement-free up to 6 months.
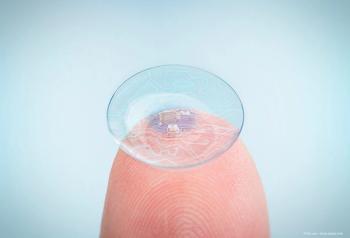
The smart contact lens is designed to offer a non-invasive solution for patients suffering from keratoconus, corneal irregularities, photophobia, and presbyopia.