
The iDose TR, a micro-invasive intraocular implant designed to lower IOP in patients with open-angle glaucoma or ocular hypertension, has a PDUFA date of December 22, 2023.

The iDose TR, a micro-invasive intraocular implant designed to lower IOP in patients with open-angle glaucoma or ocular hypertension, has a PDUFA date of December 22, 2023.
According to the company, clinical trials of the ophthalmic gel showed that patients treated with IHEEZO did not require any supplemental treatment to complete the intended surgical procedure. Harrow will host several launch events at the 2023 American Society of Cataract and Refractive Surgery annual meeting being held May 5-8, 2023, in San Diego, California.

Eyecelerator kicks off today as the American Society of Cataract and Refractive Surgery will host this year’s ASCRS and ASOA Annual Meeting in San Diego, California, beginning on Friday. ViaLase Inc., Aurion Biotech among companies presenting at the annual meeting.

New and returning special events and features on tap at annual meeting
The GA Won’t Wait campaign is designed to help older adults and their families understand and recognize the symptoms of this progressive and irreversible disease.

Deborah Ferrington, PhD, shares a brief overview of her presentation on identifying the mechanism responsible for the death of the retinal pigment epithelium.
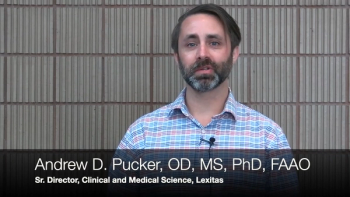
Andrew Pucker, OD, MS, PhD, FAAO, and George Magrath, MD, discuss Lexitas Pharma Services' work on modification of the National Eye Institute's corneal fluorescein staining scale.

Mark Bullimore, PhD, MCOptom, shares a brief overview of her presentation on myopia management by slowing progression with low concentration atropine.

According to the company, KPI-012 could become the first approved treatment for persistent corneal epithelial defect across all its various etiologies.
Allergan developed VUITY, which it noted is the first and only FDA-approved eye drop to treat presbyopia. The FDA has now approved a two-dose option.

The U.S. Attorney' Office Eastern District of Texas reported Kleiman Evangelista Eye Centers has agreed to pay $2,902,505 to resolve False Claims Act allegations that it offered and paid kickbacks to optometrists.

Research shows how life stressors—such as obesity—reprogram immune system cells and make them destructive to the eye as it ages.

IBI324 is a potential first-in-class ophthalmic recombinant human anti-VEGF-A and anti-Ang-2 bispecific antibody.

Trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.

Research to Prevent Blindness will launch the RPB/David L. Epstein Career Advancement Award in Glaucoma Research, sponsored by Aerie Pharmaceuticals.

The nonprofit has continually reached for new heights in the fight against avoidable blindness.

Review treatment options for both early and late complications of cataract surgery.

VUITY, developed by Allergan, is the first and only FDA-approved eye drop to treat presbyopia

Susvimo's new therapeutic approach for wet AMD may help patients maintain their vision with as few as 2 treatments per year