
Prevent Blindness is providing free geographic atrophy educational resources for patients, care partners and healthcare professionals, including a new episode of its Focus on Eye Health Expert series.

Prevent Blindness is providing free geographic atrophy educational resources for patients, care partners and healthcare professionals, including a new episode of its Focus on Eye Health Expert series.

According to the company, an additional trial is required to show positive effects on the treatment of ocular symptoms in dry eye disease.

According to the companies, the merger creates a NASDAQ-listed, late clinical-stage biopharmaceutical company focused on advancing LENZ Therapeutics’ lead assets for the treatment of presbyopia.
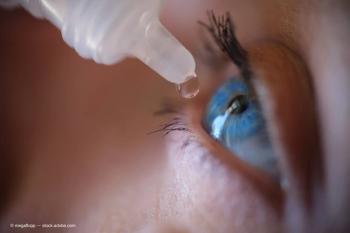
According to Sun Pharmaceutical Industries, the study design mirrors real-world experience with dry eye disease, including allowing use of artificial tears and measurement of CFS in all 5 corneal zones.
Patients who have signs or symptoms of an eye infection after using these products should talk to their health care provider or seek medical care immediately.

According to Genentech, RVO is the third indication for Vabysmo, in addition to wet, or neovascular, age-related macular degeneration and diabetic macular edema. The approval is based on two Phase III studies demonstrating early and sustained vision improvements that were non-inferior to aflibercept.

In July 2023, Harrow received certain US and Canadian commercial rights for 6 branded products from Santen.

According to a human study of older adults, grape intake improved macular pigment accumulation and downregulated harmful biomarkers.

Misusing contact lenses can be hazardous. Contact lens wearers also must take extra care when applying and removing eye cosmetics.

Ocuphire Pharma and Viatris developed the drug together for the reversal of pharmacologically-induced mydriasis (RM) produced by adrenergic agonist or parasympatholytic agents.

Research from Orbis International analyzes the overall wellbeing of children with common childhood vision problems.

According to the company, GATHER-2 24-month results met the primary objective of reducing the rate of GA growth in patients treated with IZERVAY compared to sham.
The complement C5 inhibitor significantly reduced GA progression.
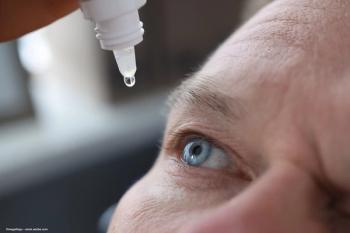
According to Bausch + Lomb, MIEBO (perfluorohexyloctane ophthalmic solution) is the first prescription eye drop that targets tear evaporation.

The FDA determined it could not approve the BLA during this review cycle due to several CMC issues, open observations from pre-approval manufacturing inspections, and a lack of substantial evidence.
According to Regeneron, the approval was based on data in the PULSAR and PHOTON trials, in which the drug demonstrated clinically equivalent vision gains to aflibercept Injection 2 mg that were maintained with fewer injections.

According to Regeneron, visual gains and safety of aflibercept 8 mg remained consistent with the established profile of aflibercept 2 mg injection.
Oculis Holding AG announced, if approved, OCS-01 has the potential to become a new standard of care as the first once-daily, topical, preservative-free corticosteroid for treating inflammation and pain following ocular surgery.

An industrial engineer and entrepreneur, George Glady was a founder of Euclid Systems Corp. and developed the concept of the Ortho-K contact lenses.

Paul Hahn, MD, PhD, shared insights on research comparing the relative efficacy of pegcetacoplan versus avacincaptad pegol in patients with geographic atrophy presented at the 2023 ASRS annual meeting.

According to the company, the therapeutic is the only approved GA treatment with a statistically significant reduction in the rate of GA progression at the 12-month primary endpoint across two phase 3 clinical trials.

According to Tarsus Pharmaceuticals, lotilaner ophthalmic solution 0.25% is the first approved therapeutic for Demodex blepharitis, and has demonstrated efficacy across multiple clinical measures of disease.

A recently completed non-human primate study of VGX-0111 demonstrated good tolerability, provided strong transgene expression in the targeted region of the retina, and increased production of the lipids whose decline is associated with macular degeneration.

Sparklers and firecrackers are at the top of the list for fireworks-related injuries. The organization is advising the public to leave fireworks to licensed operators.
The project, a collaboration with researchers from the University of Maryland, holds promise for advancing new and more tolerable drug treatments for common chronic blinding eye diseases, including glaucoma and macular degeneration.

The move could result in changes to guidelines that currently prohibit cornea donations from gay or bisexual men who have had sex with another man in the last 5 years of their life. There's no indication as to whether other queer communities—such as transgender women or nonbinary donors—will be impacted.

Instrumentation recreates properties of the myopic eye to test lenses that prevent visual decline.

According to the company, the study has now reached its goal of 300 patients enrolled to evaluate ILUVIEN as a first line, baseline therapy for DME.

Lori Pacheco, RN, CRNO, discussed sports-related eye injuries and how to prevent them at the 2023 ASCRS annual meeting in San Diego.

Mydcombi is the first FDA-approved, fixed-combination of tropicamide and phenylephrine for the reversal of mydriasis.