Dry Eye
Latest News
Video Series

Latest Videos
Shorts

Podcasts
CME Content
More News

HUC1-394 is a peptide-based eye drop for dry eyes being developed by the company.

This year's conference brought eye care professionals insights, tips, and pearls.

Researchers performed a secondary analysis of the DREAM study data.

The latest updates on myopia management, contact lenses, and dry eye were discussed in Boston.

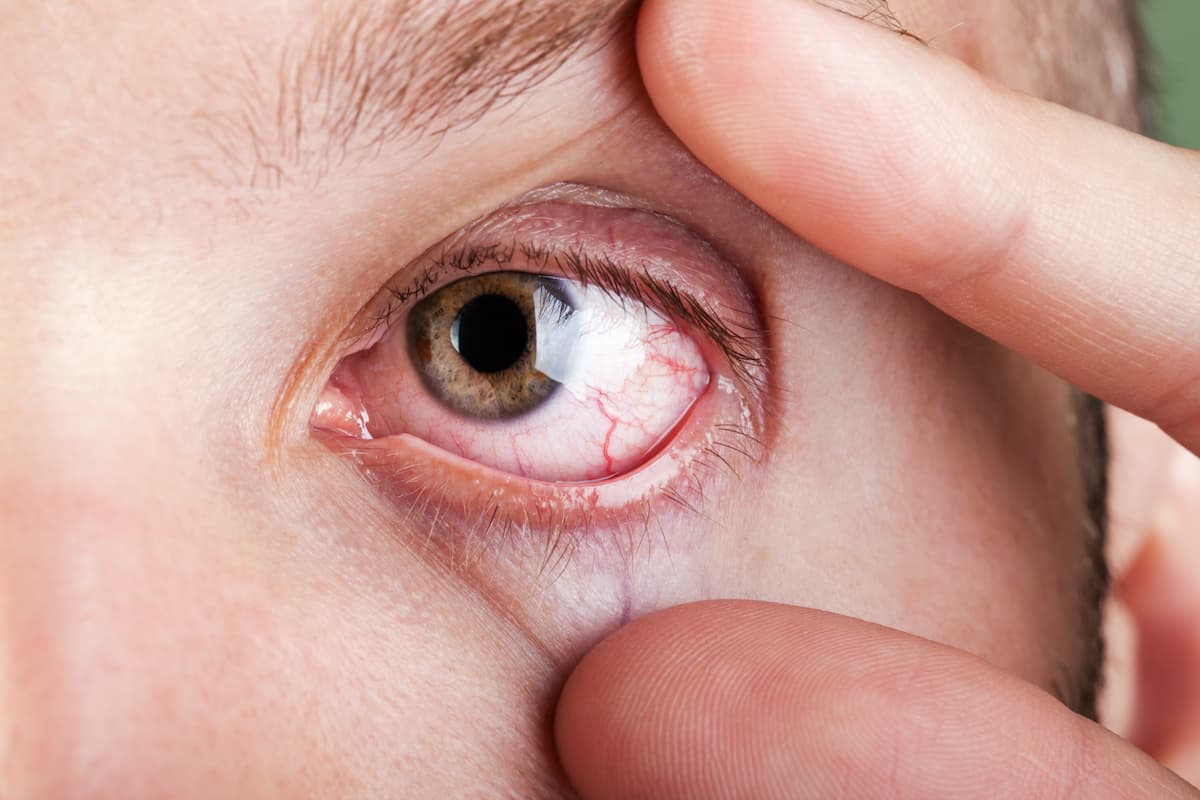
Dry eye disease is ubiquitous, with up to 20% of middle-aged patients estimated to have moderate to severe symptoms or searching for a treatment.

A focus on driver-based treatment provides a broader clinical framework for smarter, more personalized dry eye care.

Transforming care by integrating systemic, ocular, and emotional health can deliver personalized treatment and better outcomes.

Xiidra is a prescription eye drop used to treat the signs and symptoms of dry eye disease (DED) and works by targeting the source of inflammation.

Keeping up with dry eye: Approaches to treatment plans, patient education, and clinical developments
Dry eye specialists weigh in on what role dry eye plays in their practices.

New findings confirm TearCare's long-term effectiveness for dry eye disease, showing significant patient improvements with minimal treatments over 2 years.

TearCare is associated with greater health utility over time but also resulted in significant cost savings compared with CsA.
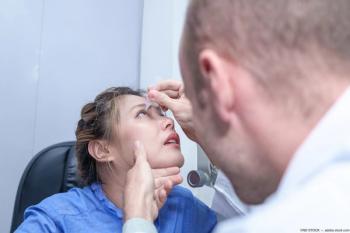
Due to ubiquity, optometrists tend to undertreat patients with dry eye.
The solution was approved by the US Food and Drug Administration in late May 2025.

The expanded clinical suite at Stanton Optical will include keratography, optical coherence tomography, and personalized therapy options for patients with dry eye.

The tool is designed to help with the application of mono-dose, dry eye eye drop vials more easily and accurately.

Ophthalmologists report high satisfaction with lifitegrast for dry eye treatment, noting rapid symptom relief and effective management in diverse patient populations.

A Prescription Drug User Fee Act (PDUFA) target action date of December 16, 2025, was assigned by the FDA.

The Topical Sustain Release drop technology was designed to address suboptimal patient adherence and high treatment cost.
The campaign, Eyes Tell the Story: The Impact of Dry Eye, will work to educate the public about dry eye by means of personal stories and survey data.

In addition to Prevent Blindness’ usual free resources, the organization is providing a dedicated webpage, fact sheets, and graphics in both English and Spanish.

Aldeyra resubmits NDA for reproxalap, aiming to address FDA concerns and demonstrate efficacy in treating dry eye disease symptoms.
Both preservative-free, contact lens-friendly drops will be available in most national retailers.

According to the company, results showed “a meaningful clinical improvement in ocular surface health” and significance for both staining end points at day 15.

Alcon’s dry eye candidate acoltremon 0.003% is a first-in-class thermoreceptor agonist, which stimulates corneal sensory nerves to increase natural tear production to treat the signs and symptoms of dry eye disease.

Bridgitte Shen Lee, OD, FAAO, FBCLA, FEAOO, discussed research that utilized IQVIA longitudinal prescription claims data from September through November 2023, coinciding with Miebo's FDA approval in September.