Dry Eye
Latest News

Latest Videos

CME Content
More News

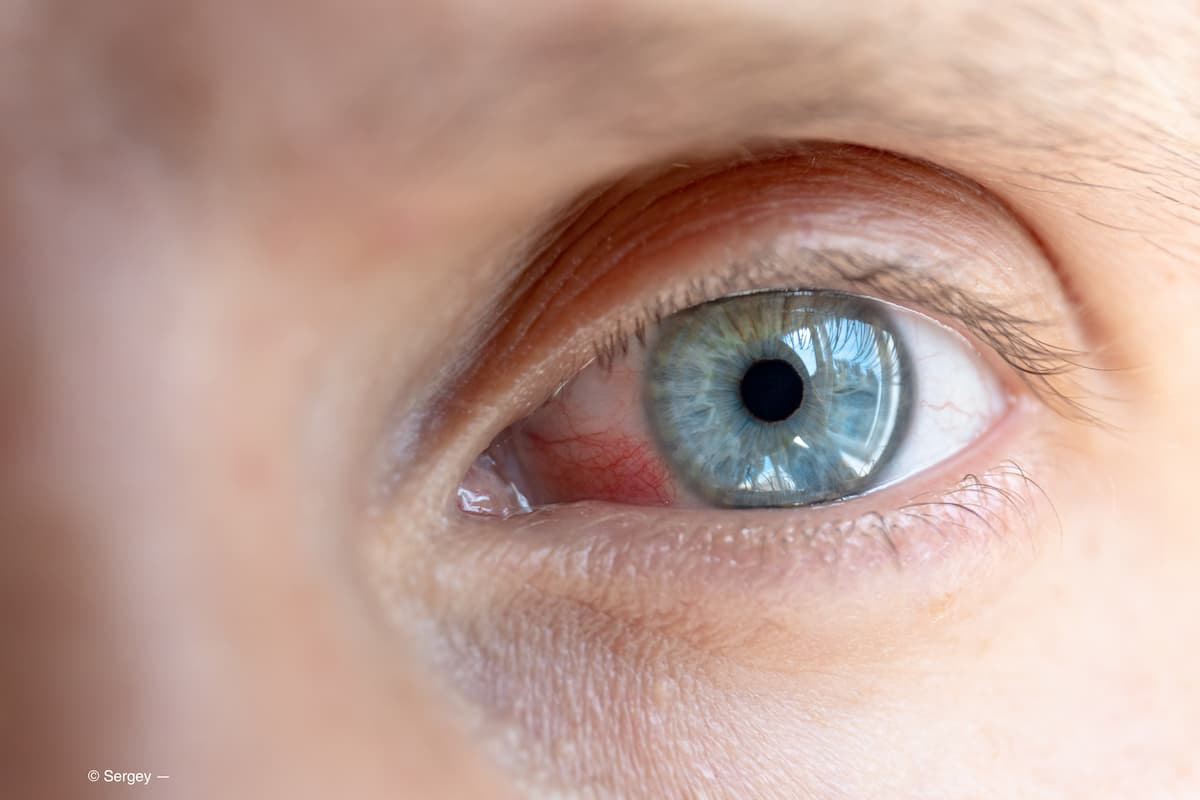
Managing dry eye disease often is key to patient comfort.

Combining simple and advanced methods can lead to optimal patient outcomes.

Approaching dry eye disease holistically will empower patients to take charge with personalized home health solutions.

Proper management results in better expectations, outcomes for patients.
Prevent Blindness aims to educate the public on dry eye through free resources including fact sheets and social media graphics for Dry Eye Awareness Month.

The company has entered a definitive agreement with Novartis to acquire Xiidra (liftegrast ophthalmic solution 5%), representing a significant opportunity for growth in prescription dry eye.
Marjorie Rah, OD, PhD, FAAO, shares brief highlights from her 2023 AOA ePoster, "Clinical performance of unique preservative-free contact lens lubricating and rewetting drop."

Erich A. Hinel, OD, MS, FAAO, Dipl ABO, discusses the use of 3-layer amniotic membrames to treat severe keratoconjunctivitis sicca, severe dry eye, and ocular surface disease.

Ahmad M. Fahmy, OD, FAAO, Dipl ABO, gives highlights of his AOA 2023 poster that was a pooled analysis of the Gobi and Mojave studies relevant to perfluorohexyloctane (NOV03) for dry eye disease associated with Meibomian gland dysfunction.

This 3-pronged approach for nutritional intervention to help maintain dry eye disease is in addition to a recommended dietary intake of daily oily fish.

When dry eye treatments are not working for your patient’s symptoms or the anterior segment exam looks normal, you must ask yourself: What if the symptoms are secondary to a neurologic dysfunction in pain perception?
Bruder Healthcare expands their dry eye portfolio by entering a distribution agreement with AllerFocus for its Percutaneous Allergy Test.

The agreement includes rights to develop, manufacture and commercialize NOV03 in Japan for the treatment of dry eye disease.

The water-free, preservative-free, anti-inflammatory drop from Novaliq, now coined Vevye, received FDA approval to treat the signs and symptoms of dry eye disease.

Since April 2022, the Dry Eye Foundation has been voicing concerns over the packaging of preservative-free drops as well as availability of noncompliant drops at online retailers.

This single-ingredient tear stabilizer from Bausch + Lomb and Novaliq could become the choice therapeutic for dry eye disease.

MIEBO, previously known as NOV03, is the first and only FDA-approved treatment for dry eye disease that targets tear evaporation.

Lucie Moore, BSc, in a presentation at the American Society of Cataract and Refractive Surgery annual meeting in San Diego, said the study focused on identifying factors that could put patients at a higher risk for developing dry eye disease.

Data on Palatin’s formulation PL9643 indicates clinical efficacy across multiple signs and symptoms of dry eye disease without sacrificing safety and tolerability.

Help patients establish a daily eye care routine like the one they have for oral health.

In addition to these tips on preventing digital eye strain, it is important to implement other eye-healthy habits.

The authors conducted a prospective non-interventional study in which a self-questionnaire was used to evaluate the ocular symptoms and medical background of patients with KC and OSD who engage in chronic eye rubbing.
An important take-away was the total and central corneal staining score improving after only 2 weeks of treatment.

Recommending a nighttime eye mask and ear plugs may protect the ocular surface.

Dirty equipment and sterilization issues are among the many violations found by the FDA.