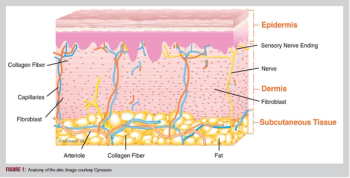
Lid and Lash
Latest News
Latest Videos
CME Content
More News

The authors concluded that patients with autoimmune hyperthyroidism who used cannabis had a significantly increased risk for TED outcomes in the 1-year interval.

A recent study assessed the effect of a modified TCLB procedure that added deframing and decompression maneuvers to the LOFC and its support structures to obtain better results for both the lower and upper lids.
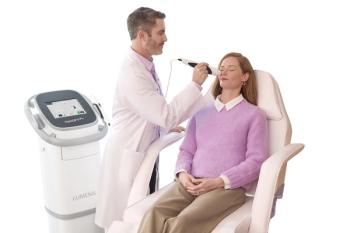
The dynamic muscle stimulation device is a noninvasive option for patients experiencing lower lid laxity and impaired blinking.

According to Mile Brujic, OD, FAAO, low-level light therapy (LLLT) and intense pulsed light have demonstrated tremendous benefits for his patients.
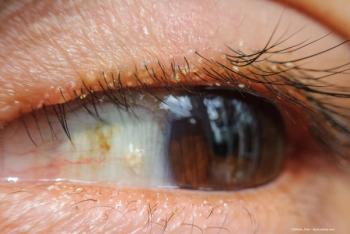
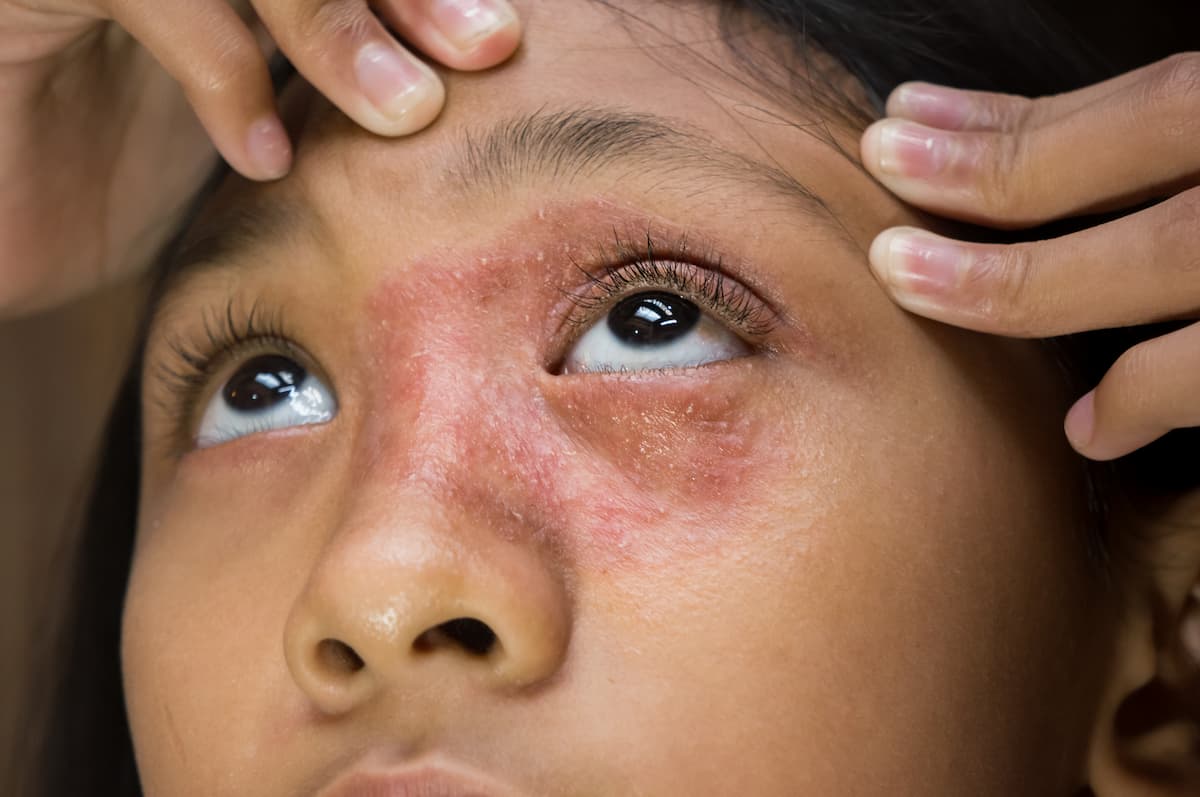
Inside the role of blepharitis in ocular surface disease and effective management strategies.

The lid wipe contains tea tree oil, hyaluronic acid, and other antibacterial and antimicrobial ingredients like euphrasia, chamomile, and rhamnose
Taking a thorough history and evaluating pertinent positives and negatives are always critical in diagnosing and treating these cases.

Perfluorohexyloctane may slow evaporation to reduce dry eye.

The silicone hydrogel daily disposable lenses contain a blend of osmoprotectants, electrolytes, and moisturizers to provide stable vision and keep eyes comfortable for 16 hours.

UC Berkeley’s Herbert Wertheim School of Optometry and Vision Science integrated the new laser in the beginning of March in its dry eye clinic for treatment of meibomian gland dysfunction.

TP-03 (lotilaner ophthalmic solution, 0.25%) was approved by the FDA in 2023 under the brand name Xdemvy for the treatment of Demodex blepharitis and is being evaluated as an investigational therapy for the treatment of Meibomian Gland Disease (MGD) in patients with Demodex mites.

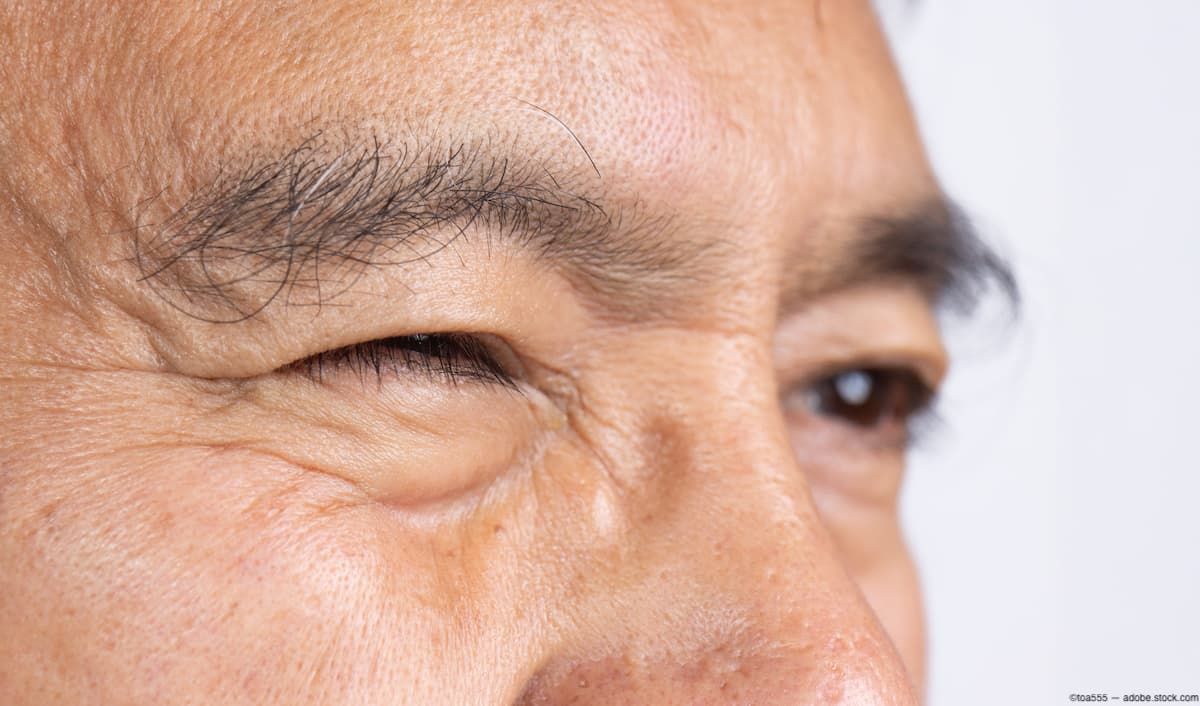
Noninvasive treatments can help patients with ptosis improve their visual field.
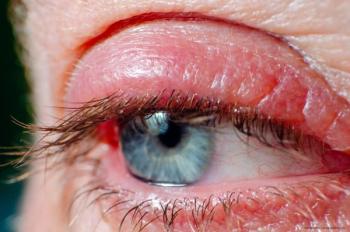
Lotilaner ophthalmic solution 0.25% (Xdemvy) was approved by the FDA in July, making it the first and only approved treatment for Demodex blepharitis.
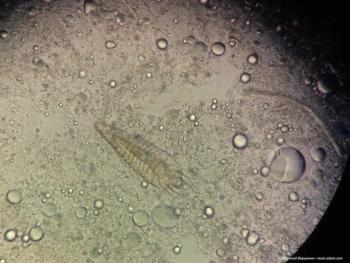
A review of Demodex mites.

BREAKING NEWS: FDA approves lotilaner ophthalmic solution 0.25% for treatment of Demodex blepharitis
According to Tarsus Pharmaceuticals, lotilaner ophthalmic solution 0.25% is the first approved therapeutic for Demodex blepharitis, and has demonstrated efficacy across multiple clinical measures of disease.

Horizon Therapeutics announced the Brazilian Health Regulatory Agency (ANVISA) has approved TEPEZZA as the first and only medicine approved in Brazil for treatment of thyroid eye disease.

Cecelia Koetting, OD, FAAO, shares insights from her AOA 2023 presentation on Demodex diaries.

The results showed that current smokers had a greater 5-year cumulative probability, compared with patients who had never smoked, of undergoing orbital decompression, strabismus surgery, and eyelid recession.

Based on the initial ophthalmic findings, it is difficult but important to predict the patients’ progression to a specific level of disease severity.

The device was designed to allow consumers to better view lids and lashes

An ocular wellness regimen doesn’t need to be complicated—simply wipe, spray, and warm