
Good safety profile and “meaningful improvement” in vision in patient with Leber congenital amaurosis.

Good safety profile and “meaningful improvement” in vision in patient with Leber congenital amaurosis.

A Prescription Drug User Fee Act (PDUFA) goal date of August 27, 2025, was set by the organization.

The decision to discontinue the trials comes shortly after the announcement that COAST missed its primary endpoint.

Paul Karpecki, OD, FAAO, sits down to discuss Sydnexis's atropine formulation and what an FDA approval would mean for myopia management.

If executed, the reverse merger would create a new ophthalmic company that pairs two FDA-approved technologies for use in glaucoma patients.

This follows successful Phase 1 results, which demonstrated a favorable safety profile for BI 771716 across both single and multiple intravitreal doses.
The new device from Lumenis aids in ocular surface disease treatment by combining radio frequency and dynamic muscle stimulation to improve lower lid laxity, enhance blink dynamics, and offer a non-invasive solution for dry eye management.


OCU410 is a novel multifunctional modifier gene therapy candidate that targets multiple pathways associated with GA.

The FDA has set a PDUFA target action date of October 23, 2025, for the low-dose atropine formulation.

ENCELTO is the first and only FDA-approved treatment for MacTel.

The gel was investigated in a case series, which was presented in a poster at SECO 2025.

Systane PRO preservative-free is the longest lasting dry eye drop in the Systane portfolio, according to Alcon.

If approved, Epoxia will be the first epi-on corneal crosslinking on the market.

The new first-line laser treatment for glaucoma and ocular hypertension will be unveiled at the 2025 American Glaucoma Society annual meeting in Washington, DC, from February 26 to March 2.
MonacoPro, the next evolution of Monaco from Optos, retains the powerful ultra-widefield SLO and spectral domain imaging while adding additional key product features.

LL-BMT1 is a sustained drug delivery approach via a 3D-printed, drug-eluting contact lens to be used in glaucoma care that successfully completed a Phase 2b clinical trial.

The approval follows a refiling after the FDA issued a Complete Response Letter due to language on the amended label.

Susvimo 100 mg/mL for intravitreal use via ocular implant is a refillable implant surgically inserted into the eye during a one-time, outpatient procedure, continuously delivering a customized formulation of ranibizumab over time.

EssilorLuxottica’s Nuance Audio glasses, which are equipped with hearing aids, will be available over the counter in the US and Europe as soon as Q1 2025.

OCU400 demonstrated meaningful improvement of 2-line gain (10 letters on ETDRS chart) in low-luminance visual acuity (LLVA) in treated eyes when compared to untreated fellow eyes.
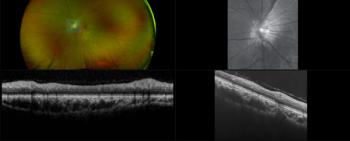
Results showed that at 6 months post-surgery, at least 1-, 2-, and 3-line gains in best-corrected distance were achieved in 97.1%, 68.6%, and 51.4% of operated eyes, respectively.

With this addition, Zeiss completes the Corneal Refractive Workflow, which already includes Visumax 800 and SMILE pro.

This year was brimming with advancements in eye care.

If approved, Glaukos states that Epioxa would be the first FDA-approved, non-invasive corneal cross-linking therapy that does not require removal of the corneal epithelium.

Aurion Biotech announced that Phase 1/2 CLARA trial of AURN001 for corneal edema demonstrated significant dose-dependent efficacy, especially in the high-dose group, with favorable safety and tolerability profiles.

A few optometrists share which innovations in optometry they were most excited about in 2024.
The dry eye drug has been assigned a PDUFA decision date of April 2, 2025, and the Aldeyra has chosen to expand their exclusive option agreement with AbbVie.
74% of participants dosed with LNZ100 achieved 3 or more lines of improvement 3 hours post-treatment.

The study looked at bevemipretide, which targets the inner mitochondrial membrane where it reversibly binds to cardiolipin.