
Phentolamine ophthalmic solution 0.75% is a preservative-free, stable eye drop, which blocks the α1 receptor within the iris dilator muscle without affecting the ciliary muscle.

Phentolamine ophthalmic solution 0.75% is a preservative-free, stable eye drop, which blocks the α1 receptor within the iris dilator muscle without affecting the ciliary muscle.

LL-BMT1 is a novel, preservative-free, weekly drug-eluting contact lens created by the company’s proprietary 3D printing technology.

The approval marks the first new steroid on the ophthalmic market in more than 15 years.

Diffusion Optics Technology (DOT) spectacle lenses are intended to slow myopia progression in children via contrast management mechanism of action.
OKYO Pharma announced the acceptance of OK-101 as an investigational new drug following clinical studies investigating the candidate in patients with neuropathic corneal pain, the first to do so.

OCULUS’ new perimetry device allows for more patient autonomy and easy data compilation for eye care providers by providing visual field tests via a virtual reality headset.

Harrow’s Vevye is the first and only cyclosporine-based product approved for the treatment of both signs and symptoms of dry eye disease.

AR-15512 is a topical transient receptor potential melastatin 8 (TRPM8) agonist designed to treat the signs and symptoms of DED via increased tear production.
According to OKYO Pharma, the first-in-human, randomized, double-masked, placebo-controlled trial of OK-101 “established a clear and informed path for further development in Phase 3 registration trials.”

The TENEO excimer laser platform is intended for use for LASIK vision correction surgery for myopia and myopic astigmatism.

According to Ocuphire Pharma, phase 3 startup activities are underway, and the clinical trial is expected to start in the first quarter of 2024.

A review of the most important content from Europe throughout 2023.

Eye care witnessed a transformative year with 11 FDA approvals. As the year concludes, there remains a robust pipeline of drugs, setting high expectations for continued advancements in ophthalmological care in 2024 and beyond.

iDose TR, a micro-invasive intraocular implant designed to lower IOP in patients with open-angle glaucoma or ocular hypertension, has received FDA approval following a new drug application (NDA) submission.

OCU410 is a modifier gene therapy product candidate being developed by Ocugen for dry AMD.

The study of OliX Pharmaceuticals' investigative property saw encouraging results and helped to identify suitable dosing levels for future clinical trials.

TP-03 (lotilaner ophthalmic solution, 0.25%) was approved by the FDA in 2023 under the brand name Xdemvy for the treatment of Demodex blepharitis and is being evaluated as an investigational therapy for the treatment of Meibomian Gland Disease (MGD) in patients with Demodex mites.

The company announced key secondary endpoints were achieved with both EYP-1901 doses. These include a more than 80% reduction in treatment burden, with nearly two-thirds of eyes supplement-free up to 6 months.

Hear about the new innovations on the market—and coming soon—from Alcon.


Qlosi (Orasis Pharmaceuticals) approved to treat presbyopia.
Aldeyra Therapeutics encounters a significant setback, with its stock value plummeting nearly 70%. The FDA indicates a potential Complete Response Letter (CRL) for reproxalap, a dry eye treatment, despite a scheduled Prescription Drug User Fee Act (PDUFA) date of November 23, 2023.

CSF-1 (0.4% pilocarpine HCl), a presbyopia-correcting drop candidate from Orasis, demonstrated consistent pupil restriction, which also suggests neuroadaptation in patients with presbyopia to the drop over time.
Bausch + Lomb is gearing up for the American Academy of Optometry (AAOpt) annual meeting, which is poised to convene in New Orleans from Oct. 11-14, 2023.

Hear from CooperVision's Michele Andrews, OD, on the latest innovations and expansions in the pipeline.

Results of the BRIO-I study, the first of 2 phase 3 clinical trials of Brimochol PF, a topical, fixed-dose combination for the treatment of presbyopia, have recently been released.

Ocuphire Pharma and Viatris developed the drug together for the reversal of pharmacologically-induced mydriasis (RM) produced by adrenergic agonist or parasympatholytic agents.

According to the company, GATHER-2 24-month results met the primary objective of reducing the rate of GA growth in patients treated with IZERVAY compared to sham.
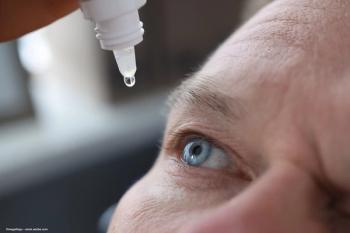
According to Bausch + Lomb, MIEBO (perfluorohexyloctane ophthalmic solution) is the first prescription eye drop that targets tear evaporation.
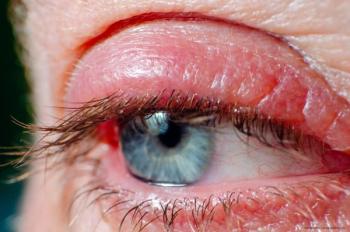
Lotilaner ophthalmic solution 0.25% (Xdemvy) was approved by the FDA in July, making it the first and only approved treatment for Demodex blepharitis.