
New long-term findings illuminate the future of DIMS spectacle lenses.

New long-term findings illuminate the future of DIMS spectacle lenses.

The FDA comments in the CRL relate to proposed labelling language, not safety, Astellas said in a press release.
The Finnish company's new device sports a universal adapter and rebound tonometry technology.

The RayOne Galaxy and Galaxy Toric IOL will launch at this year's ESCRS Congress in Barcelona, Spain.

Aflibercept-jbvf and aflibercept-yszy are the first interchangeable biosimilars to aflibercept 2 mg that have been approved in the US.

David A. Berntsen, OD, PhD, FAAO, talks through his 2024 ARVO presentation on the BLINK2 study and the findings on axial growth after discontinuing soft multifocal contact lens wear.

Before the ASCRS 2024 meeting, TheiaNova CEO Carissa Fonseca, PhD, pitched a novel keratoconus treatment.
Nicholas Gilberg, OD, with EssilorLuxxotica's Leonardo team walks through the basics of the education software that can be implemented in any practice.


Laura Periman, MD, talks through what was gained from the conference, from becoming acquainted with new technological advancements to renewing passion for dry eye care.

Scott Moscow, OD, talks his practice's contact lens "test-driving" option and how to get patients on board with trying out new corrective eyewear.

Alysse Henkel, vice president of research and inSights at The Vision Council, details recent research conducted by the company regarding consumer purchasing habits and trends.

Filomena Ribeiro, MD, PhD, FEBO, discusses the crucial role of mentors – and where to find them.

The approval marks the first new steroid on the ophthalmic market in more than 15 years.

A review of the most important content from Europe throughout 2023.
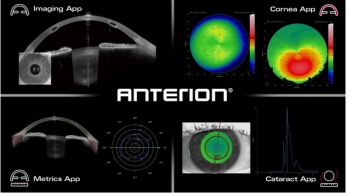
The ‘all-in-one’ platform combines specialized imaging and three new FDA-approved apps with a focus on metrics, corneal imaging, and cataract care.

The non-profit will partner with the imaging company to prevent blindness and fund new retinoblastoma research.