
Fully recovered COVID-19 patients have a higher prevalence for developing dry eye disease, researchers report.

Fully recovered COVID-19 patients have a higher prevalence for developing dry eye disease, researchers report.
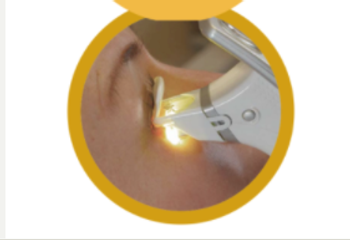
As an all-in-one handheld device, eyecare practitioners can tailor patients’ needs for MGD treatment in both eyes in approximately 8 to 12 minutes.

New research finds a drug used to treat alcohol use disorder could be a game-changer in restoring vision for patients with progressive blinding disorders.
This latest approval for Beovu (brolucizumab) 6 mg is the second indication granted by the EC, as it was first approved in 2020 for the treatment of AMD.

Heru Chief Marketing Officer Brandon Barber speaks on the company's upcoming advancements and two new testing modalities on its diagnostic platform: Fast Pattern Suprathreshold Visual Field and the Farnsworth D-15 extended color vision exam.

Mark Dunbar, OD, FAAO, offers a look at what to expect from this year's high-sakes competition at Vision Expo East.

An exciting line-up of speakers and panelists is promised for the main-stage destination at VEE 2022.

Approved by the FDA last October, XIPERE (triamcinolone acetonide injectable suspension) was approved by the FDA last October as the first and only therapy for treating macular edema associated with uveitis.

US commercialization of EVO will begin immediately in select cities across the country.
The latest launches of BRIO-I and BRIO-II follow promising topline data from the phase 2 VIVD clinical study evaluating Brimochol PF.

With its portable capabilities, the Heru platform replaces legacy diagnostic devices and surpasses standard care in cost and size.

Originally postponed due to COVID-19, the annual event will now be held this June at the JW Marriott Grande Lakes in Orlando, Florida.
CSF-1 is a preservative-free solution designed as an alternative to reading glasses for patients.

The company expects to enroll patients diagnosed with keratoconus in two trials at clinical sites in the US, South America, Europe, and Asia.
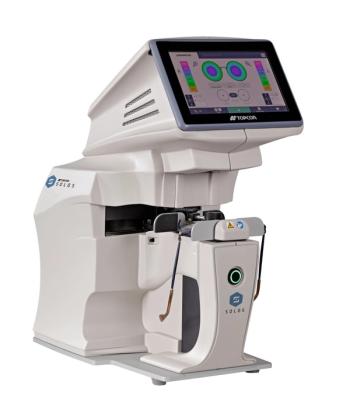
SOLOS is automatic lens analyzer that enables advanced, accurate, and efficient lens analysis with one button touch.
Daniel Fuller, OD, FAAO Dipl., FSLS, discusses augmenting corneal thickness in thin keratoconus corneas during corneal cross-linking via FDA-approved hypotonic riboflavin during SECO 2022.

The unique partnership and corresponding sponsorship is the largest in ASCO’s history.
Mohammad Rafieetary, OD, FAAO, offers a sneak peek at the latest (and fake!) news in retina.
Mohammad Rafieetary, OD, FAAO, shares highlights from his SECO 2022 presentation titled "Image-in that! Scanning for retinal disease."
Selina McGee, OD, FAAO, disscusses her SECO 2022 presentation "See the light! Adding IPL to your practice."
Kelly Voltz, OD, FAAO, FSLS, on highlights from her presentation "Ortho-K" during the 2022 SECO meeting.

The purchase gives Théa ownership over seven products across the therapeutic space, including glaucoma and ocular surface disease.

Effective this month, the coverage applies to approximately 70% of all United Healthcare commercial lives.

Daily disposable contact lenses combine with an established antihistamine for the world's first drug-eluting contact lenses.
Topline proof-of-concept data are expected by the first quarter of 2023.

In the past 12 months, the program has prevented the equivalent of 28 million plastics bottles from polluting the oceans.


TRYVAYA is the first and only FDA-approved nasal spray for the treatment of dry eye disease.
Shamir joined the Alpine in May 2021 in partnership with Alpine F1 Team to enhance the visual quality and safety of its race team members.
Optometry Divas' founder Lauretta Justin, OD, shares a sneak peek at the agenda for the organization's annual educational retreat "Discover the Magic," this summer.