
Optometry Times Staff Reports
Articles by Optometry Times Staff Reports


Nominations for the 2023 awards will close July 31, 2023.

The National Keratoconus Foundation has named NASCAR driver Joey Gase as Ambassador.

The annual conference will take place September 6-9 at the Renaissance Schaumburg Convention Center in Schaumburg, IL.

The organization now has chapters in Boston, San Antonio, New York, and Los Angeles.

Latinos En Optometry, also known as LEO, is launching and wants you to join their community as they expand membership, sponsorship, events, and engagement in the year ahead.


Kicking off AMD Awareness Month, give us your thoughts!

Essence Johnson, OD, FAAO, Dipl ABO, has been appointed as the first Black EyeCare Perspective executive director.

The positive 3-month efficacy and safety results from Azura Ophthalmic's Phase 2b study of AZR-MD-001 0.5% in Meibomian Gland Dysfunction (MGD) met its co-primary endpoints.

This acquisition for Alcon means a growing ophthalmic portfolio of commercial products and development pipeline.

In honor of World Sight Day, optometrists and ophthalmologists reflect on what today means to them.

The event is part of the organization's pipeline for Black students into optometry.

Vizient, Inc., designates NYU Langone as number 1 for patient care.
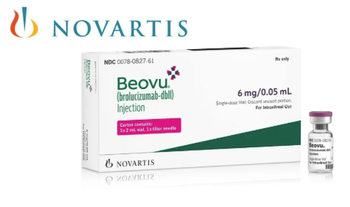
This latest approval for Beovu (brolucizumab) 6 mg is the second indication granted by the EC, as it was first approved in 2020 for the treatment of AMD.

An exciting line-up of speakers and panelists is promised for the main-stage destination at VEE 2022.

Approved by the FDA last October, XIPERE (triamcinolone acetonide injectable suspension) was approved by the FDA last October as the first and only therapy for treating macular edema associated with uveitis.

US commercialization of EVO will begin immediately in select cities across the country.
The latest launches of BRIO-I and BRIO-II follow promising topline data from the phase 2 VIVD clinical study evaluating Brimochol PF.

With its portable capabilities, the Heru platform replaces legacy diagnostic devices and surpasses standard care in cost and size.

Originally postponed due to COVID-19, the annual event will now be held this June at the JW Marriott Grande Lakes in Orlando, Florida.
CSF-1 is a preservative-free solution designed as an alternative to reading glasses for patients.

The company expects to enroll patients diagnosed with keratoconus in two trials at clinical sites in the US, South America, Europe, and Asia.
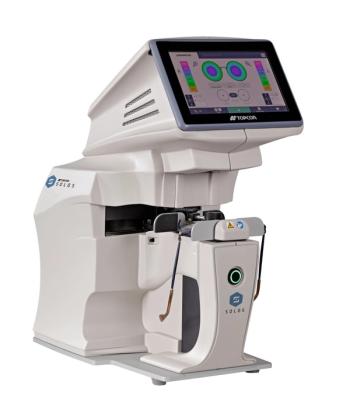
SOLOS is automatic lens analyzer that enables advanced, accurate, and efficient lens analysis with one button touch.

The unique partnership and corresponding sponsorship is the largest in ASCO’s history.

The purchase gives Théa ownership over seven products across the therapeutic space, including glaucoma and ocular surface disease.

Effective this month, the coverage applies to approximately 70% of all United Healthcare commercial lives.

Daily disposable contact lenses combine with an established antihistamine for the world's first drug-eluting contact lenses.
Topline proof-of-concept data are expected by the first quarter of 2023.

In the past 12 months, the program has prevented the equivalent of 28 million plastics bottles from polluting the oceans.
Latest Updated Articles
 Théa to acquire 7 branded ophthalmic products from Akorn
Théa to acquire 7 branded ophthalmic products from AkornPublished: January 29th 2022 | Updated:
 ASCO, Johnson & Johnson Vision partner for more diversity in optometry
ASCO, Johnson & Johnson Vision partner for more diversity in optometryPublished: March 12th 2022 | Updated:
 Shamir Insight launches two new, updated lens innovations
Shamir Insight launches two new, updated lens innovationsPublished: February 2nd 2022 | Updated:
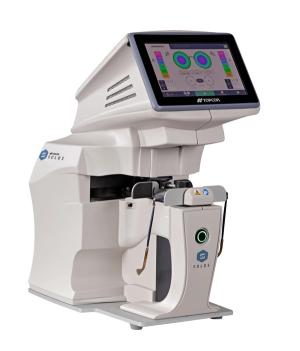 Topcon introduces SOLOS Automatic Lens Analyzer
Topcon introduces SOLOS Automatic Lens AnalyzerPublished: March 13th 2022 | Updated:
 Lumenis partners with Mandy Moore on DED awareness
Lumenis partners with Mandy Moore on DED awarenessPublished: February 8th 2022 | Updated:
 'Open Your Eyes' documentary to premiere this month
'Open Your Eyes' documentary to premiere this monthPublished: January 2nd 2022 | Updated:
