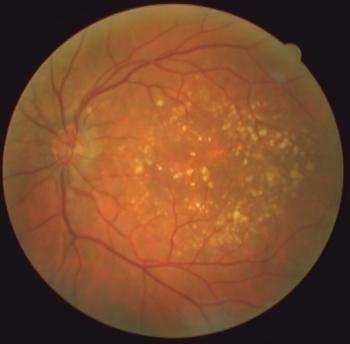
According to Genentech, RVO is the third indication for Vabysmo, in addition to wet, or neovascular, age-related macular degeneration and diabetic macular edema. The approval is based on two Phase III studies demonstrating early and sustained vision improvements that were non-inferior to aflibercept.