
A look back on what happened in optometry during the week of March 20-March 24.


A look back on what happened in optometry during the week of March 20-March 24.
Investigators evaluated risuteganib for safety and effectiveness in patients with dry AMD.
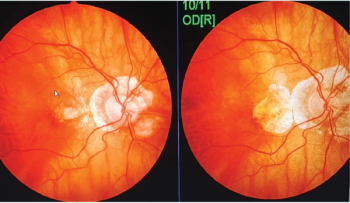
These disorders account for most of the vision loss in working-age and elderly Americans.

A look back on what happened in optometry during the week of March 10-March 16.

Research shows how life stressors—such as obesity—reprogram immune system cells and make them destructive to the eye as it ages.

A look back on what's happened in optometry during the week of February 25-March 2.
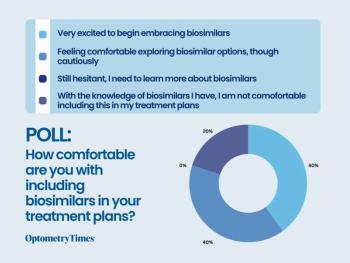
See the results from our recent poll!

A look back on what's happened in optometry during the week of February 18-February 23.
The eye care community is moving toward a more disruption-resistant system of care, from wearable tech to VEGF-eluting implants.

OPT-302 combined with ranibizumab: covering all the VEGF bases for superior visual gains compared with ranibizumab alone in wet AMD

Vote in our most recent poll!

Advances in drug creation would reduce cost burden of treatment for patients.
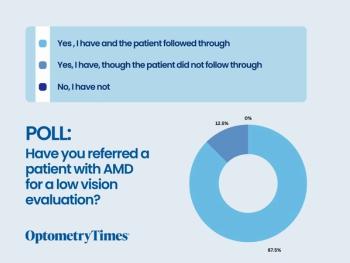
See the results from our recent poll!

The trial showed no added benefit of nesvacumab and aflibercept combination therapy over aflibercept monotherapy.

Bausch + Lomb and Modulight announce the photodynamic laser for use with photodynamic therapy was approved by the FDA for the treatment of patients with subfoveal choroidal neovascularization due to AMD.

Kicking off AMD Awareness Month, give us your thoughts!

Benefits include eliminating eyeglass problems and boosting patient confidence.

A look back on what's happened in optometry during the week of Jan. 1-Jan. 5, 2023.

As Baby Boomers enter the at-risk age group for vision loss, optometrists must be ready to act.

A multicenter US study revealed that the long-term use of metformin to treat diabetes and lifestyle changes in patients with diabetes were not associated with the risk of age-related macular degeneration (AMD).

As 2022 comes to a close, check out some of the hottest and most-liked stories from the year!
Brad Sutton, OD, FAAO, FORS, discusses key takeaways from his discussion, "Genetic testing in AMD: critical, useful, or inappropriate," which he presented during the 2022 American Academy of Optometry meeting.
Anthony DeWilde, OD, FAAO, shares highlights from his AAOpt presentation, "Anti-VEGF and the eye: Past, present and future."

World Sight Day is upon us and RestoringVision has issued a lofty but doable goal: help solve the global vision crisis.

In honor of World Sight Day, optometrists and ophthalmologists reflect on what today means to them.