Leo P. Semes, OD, FAAO, discusses his presentation on, "A new look at AMD," during the South Carolina Optometric Physicians Association's annual meeting.

Leo P. Semes, OD, FAAO, discusses his presentation on, "A new look at AMD," during the South Carolina Optometric Physicians Association's annual meeting.

Ranibizumab-eqrn will offer greater treatment access and choice for patients, payors, and providers in the US.

Korean investigators have found intermittent fasting may actually lead to a lower risk of developing AMD in the elderly population.

Presented during the 2022 ASRS meeting, results find that over 60% of participants could be treated every 4 months at 2 years—an increase from 45% at year 1.
A decade after the conclusion of the NIH-funded AREDS2 study, researchers found that the AREDS2 formula also reduces the risk of lung cancer.

Trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.

Elena Biffi, OD, MSc, FAAO, dives into AMD, a multifactorial disease that requires a multimodal diagnostic imaging approach.

Embracing this new model of care can allow optometric practices to flourish—and may enable earlier detection of conversion from intermediate dry to wet AMD.

Early diagnosis and intervention are crucial when managing this patient base.

A decision by the FDA is expected in August 2022.

The company, formerly known as Clover Therapeutics, intends to use the funding to advance two lead drug candidates that target molecularly-defined AMD patient subtypes.

The company's technology allows for ECPs to detect, monitor, and treat AMD 3 years before clinical diagnosis.
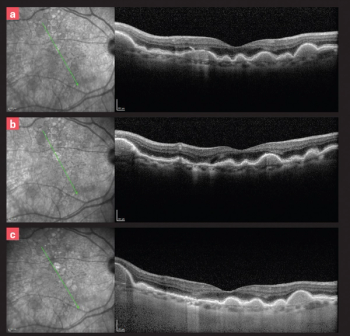
A look at the latest developments in retinal disease, including clinical trials and advanced therapies.
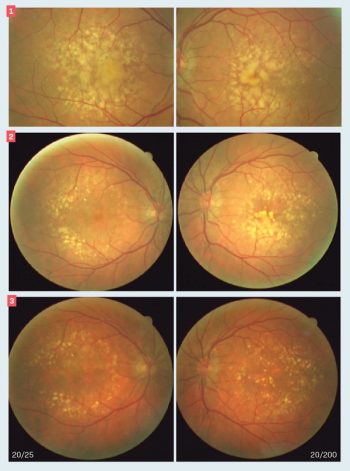
Diagnosis highlights overlooked treatments for this disease

Gifted by philanthropist James Grosfeld, the money will fund the launching of a pioneering research initiative as one of the largest investments of resources in the United States.

Researchers found that patients with nAMD lost vision as a result of the lockdown and reduced number of treatments during the COVID-19 pandemic.

Elevatum was created as a phase 4, multicenter, open-label, single-arm trial to specifically study Vabysmo (faricimab-svoa) in these underrepresented populations.

New research finds a drug used to treat alcohol use disorder could be a game-changer in restoring vision for patients with progressive blinding disorders.

Faricimab is now the first and only FDA-approved drug to target two distinct pathways known to cause retinal disease that may lead to vision loss.
Roundtable of experts discuss impact, management of retinal disease.
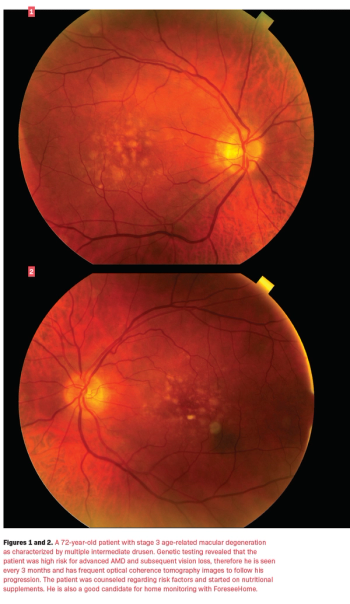
Practitioners spend more time diagnosing glaucoma or diabetic retinopathy than AMD