Ocular Surface Disease
Latest News

Latest Videos

CME Content
More News

Pamela E. Theriot, OD, FAAO, chats about the relationship between makeup and the ocular surface, and when and how how to intervene as a medical professional.

Actionable tips to improve comfort and fit.

Results suggest higher exposures to lower temperatures and certain air pollutants could result in worsened ocular symptoms and increased tear osmolarity.

Long-term vision and ocular surface health are just part of the puzzle.
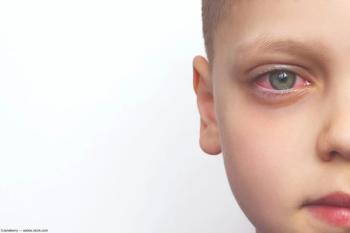
Pediatric TINU syndrome is rare, but responds well to treatment.

The survey found that most participants may not know that their symptoms could be associated with eye dryness, including redness, fluctuating vision, a scratch, gritty, tired, or heavy feeling in the eye, or overall eye irritation.

Exploring strategies for effective management of a multifaceted condition.

Jade Coats, OD, details 2 poster presentations on the benefits found in a novel lipid-containing eye drop during AOA's 2024 Optometry's Meeting.

The study also discovered a need for more blatant labeling on e-liquid containers to help reduce accidental contact with the ocular surface.
Honest discussions about treatments for dry eye disease can enhance compliance and simplify care.

Pucker shares topline results from a study that investigated the difference in meibomobian gland morphology when measured with a Visante OCT versus an OCULUS Keratograph 5M.

The nutritional supplement comprised of a proprietary blend of lutein, zeaxanthin isomers, curcumin, and vitamin D3 improved tear production, tear film break-up time, and tear osmolarity, among others.
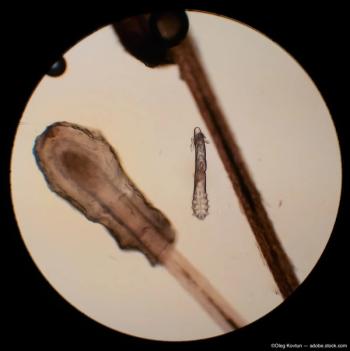
Trial findings show efficacy of lotilaner for killing mites

The optometry school, founded in 1904, was the first of its kind in California.

Phentolamine ophthalmic solution 0.75% is a preservative-free, stable eye drop, which blocks the α1 receptor within the iris dilator muscle without affecting the ciliary muscle.

Optometrists are often the first to notice ocular adverse effects from systemic medication. When should optometrists reach out to prescribing doctors?
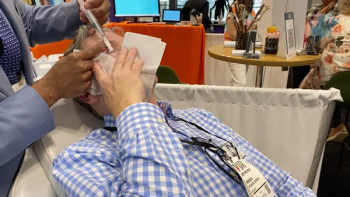
At Vision Expo East 2024, Srinivas Kondapalli, MD, demonstrates how to use Rinsada to address ocular surface disease.

Mile Brujic, OD, FAAO, speaks on the power of Rinsada to bust biofilms at Vision Expo East 2024.
OKYO Pharma announced the acceptance of OK-101 as an investigational new drug following clinical studies investigating the candidate in patients with neuropathic corneal pain, the first to do so.

Harrow’s Vevye is the first and only cyclosporine-based product approved for the treatment of both signs and symptoms of dry eye disease.
According to OKYO Pharma, the first-in-human, randomized, double-masked, placebo-controlled trial of OK-101 “established a clear and informed path for further development in Phase 3 registration trials.”

Regarding both the amount and speed of symptom improvement, patients were more satisfied with the insert compared with the topical steroid, but there was no statistically significant difference vs the topical antihistamine in terms of satisfaction.
The revision is in response to multiple recalls for eye drops and instances of consumer injury and death in 2023.

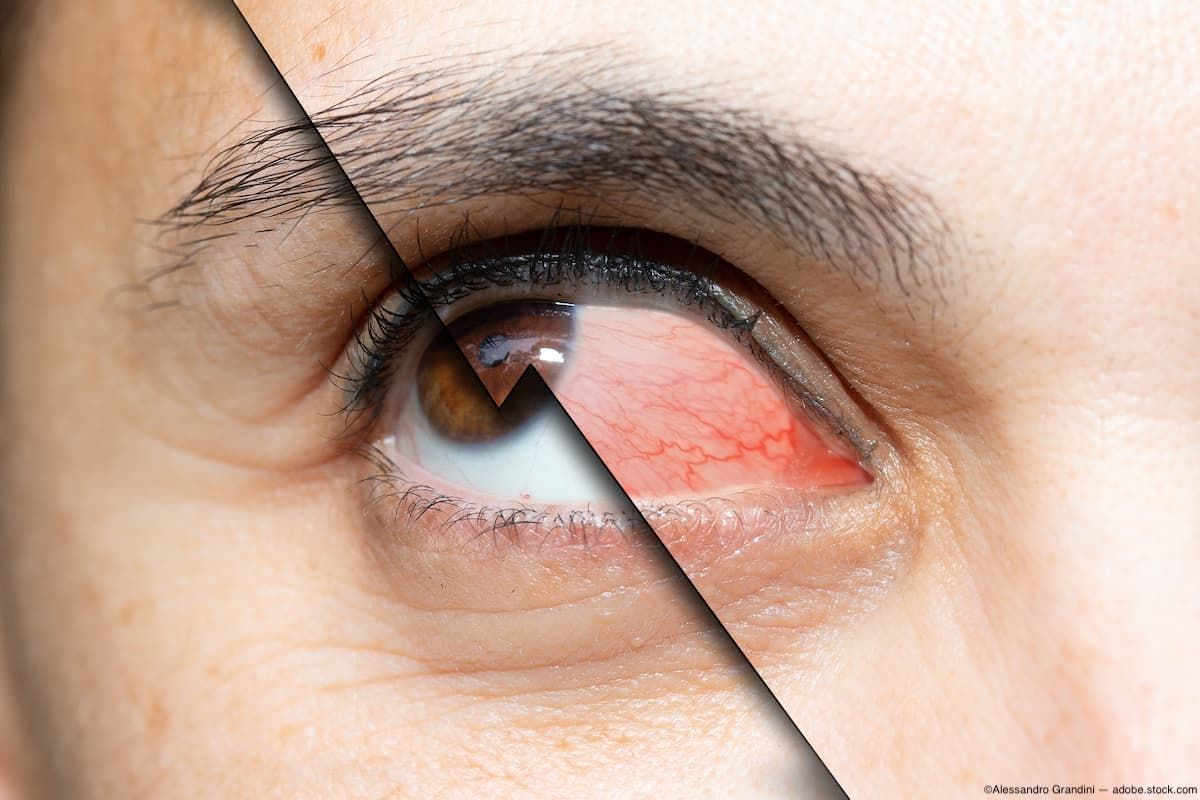
Dry eye disease incidence rates vary markedly depending on the eye care practice.

Vezocolmitide is the first drug candidate based on PolyCol, Stuart Therapeutics' patented synthesized polypeptide collagen mimetic peptide platform.