Ocular Surface Disease
Latest News

Latest Videos

More News

Much has changed in the study of keratoconus over the years.
A look back at some of the biggest stories in ophthalmology in 2023.


2 cases illustrate the importance of ocular surface optimization.

Using the NEI protocol for addressing corneal staining, researchers used 5 microliters of 2%, non preserved fluorescein and then evaluated corneal staining.

This is just one of many times the FDA has issued a warning to companies for the sale of unapproved ophthalmic products in 2023.

For patients with keratoconus-related inflammation, an intracanalicular steroid insert rivals the effectiveness of eye drops while boosting adherence.
Patients who have signs or symptoms of an eye infection after using these products should talk to their health care provider or seek medical care immediately.
The FDA recalled 26 eye drops due to risk of infection and possible vision loss.

Here’s why formulation matters.

Collaboration between ophthalmologists, optometrists can boost early detection.
Tracy Doll, OD, FAAO, shares an overview of her anterior segment symposium presented during the 2023 AAOpt annual meeting.

Private practice eye care providers’ ability to immediately administer antibiotic eye drops or have them overnighted to the patient is a giant step forward for practice and patient.

Therapies and technologies to treat allergic conjunctivitis are rapidly changing.

Hear what is new in the world of ocular surface disease, and what's coming down the pipeline.

Results of the BRIO-I study, the first of 2 phase 3 clinical trials of Brimochol PF, a topical, fixed-dose combination for the treatment of presbyopia, have recently been released.

Ben Bergo, CEO of Visus, shares data from the company's pivotal phase 3 BRIO-1 trial, as well as answers questions about Brimochol PF.

Easy-to-use modality offers exciting benefits for patients.

Combination therapy may reduce adverse events related to corticosteroids alone.

Set your young patients—and their caregivers—up for success in treatment.
According to Bausch + Lomb, MIEBO (perfluorohexyloctane ophthalmic solution) is the first prescription eye drop that targets tear evaporation.

Dual-purpose therapeutics help patients with both conditions.
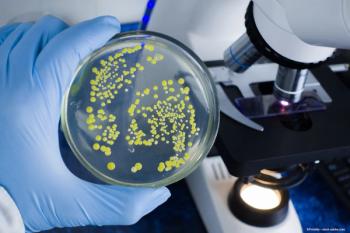
The organization says to stop using Dr. Berne’s and LightEyez MSM drops immediately due to bacterial contamination, fungal contamination, or both.

This NDA transfer marks the fourth FDA-approved ophthalmic product purchased by Harrow earlier in 2023 now commercially available in the US.
Pediatric keratoconus is often more challenging to diagnose, resulting in treatment delay and vision loss.