Retina
Latest News
Latest Videos

CME Content
More News

deepeye TPS is designed to close the gap between real-world outcomes and trial data by reducing patient dropout.
The results of an investigation into iRORA identified a wide spectrum of fundus autofluorescence patterns that corresponded with iRORA lesions and that those patterns were associated with conversion to cRORA over time.

Susvimo is the first and only FDA-approved, continuous delivery treatment shown to maintain vision in people with DR with just one refill every 9 months
New research reveals how low blood sugar can damage the retina in diabetes, highlighting potential treatments targeting specific proteins to prevent vision loss.
DREAM is an acronym for deep imaging depth, rapid sweeping speed, extensive scan range, accurate results, and multimodal imaging capabilities.

In total, 613,690 images used for screening were included in the study.
The recent study details the diagnostic value of a multimodal model utilizing both types of biomarkers when compared with unimodal models.

The system was taught quality standards by being fed more than 1400 images from different parts of the retina taken by adaptive-optics ophthalmoscopy.
The drug was previously approved for high blood pressure in 1955.

Lindsell discusses promising retinal therapeutics, such as at-home therapies, gene therapy, and devices like the Valeda Light Delivery System (LumiThera), which uses photobiomodulation and shows early promise in preventing geographic atrophy altogether.
Topline results from the MAGNIFY phase 2 clinical trial of oral zervimesine show 28.6% slower geographic atrophy lesion growth compared with placebo.

Boehringer Ingelheim announced that the phase 2 clinical studies will investigate a potential first-in-class oral compound and a highly specific antibody fragment for geographic atrophy.

Dilsher Dhoot, MD, FASRS, shares his forward-looking perspective on developments in retinal disease management, highlighting both recent breakthroughs and promising innovations on the horizon.

As a retinal expert, Ferrucci discusses some emerging retinal therapies that excite him.

With the acquisition, Topcon Healthcare also assumes RetInSight’s AI algorithms that analyze retinal images to detect and monitor disease.
The company said it will be available on the US market for use in clinics and healthcare institutions.


The optimal triple-carotenoid supports daily eye health with lutein, zeaxanthin, and meso-zeaxanthin, according to the company.
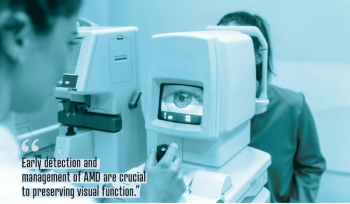

The starry faculty has a galaxy of symposia planned for May 4, 2025.
The FDA did not agree with a supplemental Biologics License Application for the addition of extended dosing intervals of up to 24 weeks across currently-approved indications.
The supplemental BLA seeks approval for Eylea HD for both the treatment of macular edema following retinal vein occlusion and for broadening the dosing schedule to include every-4-week dosing across approved indications.

CRU 2025 provided the latest insights regarding glaucoma, dry eye disease, retina, myopia, neurotrophic keratitis, and keratoconus.

Rachelle Lin, OD, MS, FAAO, and Quan Đông Nguyễn, MD, MSc, lectured on retinal updates at CRU 2025. The pair also covered the power of a low vision referral for patients with retinal disease.

Good safety profile and “meaningful improvement” in vision in patient with Leber congenital amaurosis.