Retina
Latest News
Latest Videos
CME Content
More News

Prevent Blindness is providing free geographic atrophy educational resources for patients, care partners and healthcare professionals, including a new episode of its Focus on Eye Health Expert series.

Between 2010 and 2019, this study retrospectively examined and identified all patients diagnosed with syphilitic uveitis who were admitted to inpatient facilities in the United States.
Community-based eye care is one of Orbis’s top priorities in the country.

A multicenter study in Japan examined patients to understand geographic atrophy characteristics and progression rate.

Is telemedicine through retinal images a solution to earlier intervention and increased access to care? Fortunately—and unfortunately—yes.

Counsel your at-risk patients on strategies to minimize severe hypoglycemia.

These continued studies also demonstrate a well-tolerated safety profile in a broad population of more than 1,200 patients.

According to Genentech, RVO is the third indication for Vabysmo, in addition to wet, or neovascular, age-related macular degeneration and diabetic macular edema. The approval is based on two Phase III studies demonstrating early and sustained vision improvements that were non-inferior to aflibercept.

In July 2023, Harrow received certain US and Canadian commercial rights for 6 branded products from Santen.
The good, the bad, and the questionable.
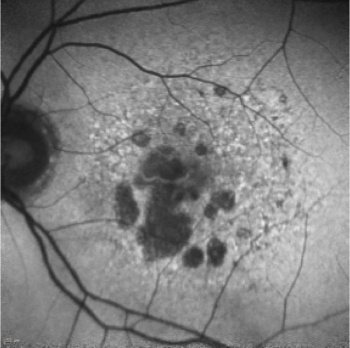
The first approved GA treatment leaves room for additional therapies.

Drs Paul Chous and Jeffry Gerson brought a new spin on the diabetes talk they've been presenting the last 18 years at the American Academy of Optometry annual meetings.

This podcast provides a review of some of the key highlights from the Controversies in Modern Eye Care meeting held in April 2023. The program is designed for those who did not attend the live meeting and to help reinforce learnings for those who did.

Steven Ferrucci, OD, FAAO, discusses imaging techniques and treatment options for geographic atrophy as part of his AAOpt presentation alongside Carolyn Majcher, OD, FAAO.

According to the company, GATHER-2 24-month results met the primary objective of reducing the rate of GA growth in patients treated with IZERVAY compared to sham.
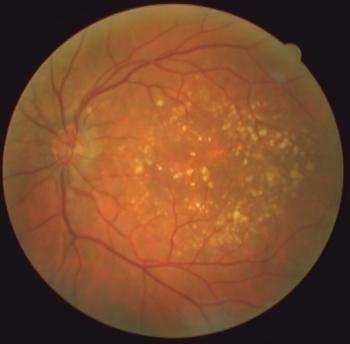
Prevention should be at the forefront of the discussion.

T1D is one of the most common chronic diseases affecting pediatric populations.

Clinicians may have difficulty in making a diagnosis.
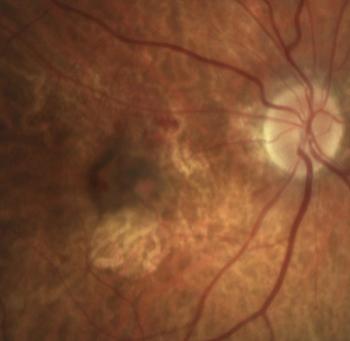
You may be wondering: What is all this hype about geographic atrophy (GA)? If you are, let us look at the enigma.
According to Regeneron, the approval was based on data in the PULSAR and PHOTON trials, in which the drug demonstrated clinically equivalent vision gains to aflibercept Injection 2 mg that were maintained with fewer injections.

Paul Hahn, MD, PhD, shared insights on research comparing the relative efficacy of pegcetacoplan versus avacincaptad pegol in patients with geographic atrophy presented at the 2023 ASRS annual meeting.

According to the company, the therapeutic is the only approved GA treatment with a statistically significant reduction in the rate of GA progression at the 12-month primary endpoint across two phase 3 clinical trials.
Data from the 30 month GALE extension study indicated that continuous treatment with pegcetacoplan injection showed increasing beneficial effects over that timeframe in patients with geographic atrophy (GA).

Unpacking the connection between type 2 diabetes and Alzheimer disease.
Opthea’s lead biologic drug candidate OPT-302, which is under investigation for the treatment of wet AMD, received nonproprietary drug name sozinibercept, which is a critical step in the FDA approval process.















































.png)


