
The company, formerly known as Clover Therapeutics, intends to use the funding to advance two lead drug candidates that target molecularly-defined AMD patient subtypes.

The company, formerly known as Clover Therapeutics, intends to use the funding to advance two lead drug candidates that target molecularly-defined AMD patient subtypes.

The company's technology allows for ECPs to detect, monitor, and treat AMD 3 years before clinical diagnosis.
A look at the latest developments in retinal disease, including clinical trials and advanced therapies.

Oxurion NV finds insufficient evidence of efficacy on key clinical endpoints for THR-687 in Part A of the INTEGRAL phase 2 trial; focus will now turn to an alternative candidate and trial.

New detailed, longer-term findings were presented at the 2022 ARVO meeting.
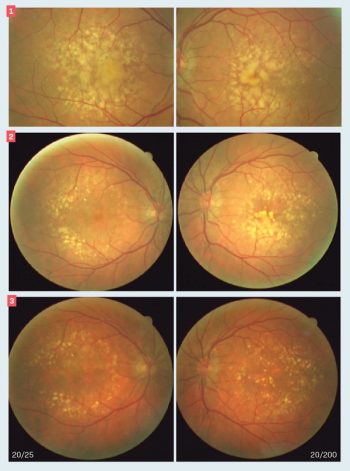
Diagnosis highlights overlooked treatments for this disease
Investigators report a correlation between the risk of 5-letter visual acuity (VA) loss at 24 months for eyes with clinically significant diabetic macular edema (CSDME) and good VA initially treated and eyes that were initially observed in routine clinical practice.

Gifted by philanthropist James Grosfeld, the money will fund the launching of a pioneering research initiative as one of the largest investments of resources in the United States.

Elevatum was created as a phase 4, multicenter, open-label, single-arm trial to specifically study Vabysmo (faricimab-svoa) in these underrepresented populations.

New research finds a drug used to treat alcohol use disorder could be a game-changer in restoring vision for patients with progressive blinding disorders.
Vascular density might be a biomarker for microvascular abnormalities following COVID-19
This latest approval for Beovu (brolucizumab) 6 mg is the second indication granted by the EC, as it was first approved in 2020 for the treatment of AMD.

Approved by the FDA last October, XIPERE (triamcinolone acetonide injectable suspension) was approved by the FDA last October as the first and only therapy for treating macular edema associated with uveitis.

SECO hosted its 2022 annual meeting March 9-13 at the Ernest N. Morial Convention Center with a LIVE CE virtual meeting available to online attendees.
Mohammad Rafieetary, OD, FAAO, offers a sneak peek at the latest (and fake!) news in retina.
Mohammad Rafieetary, OD, FAAO, shares highlights from his SECO 2022 presentation titled "Image-in that! Scanning for retinal disease."
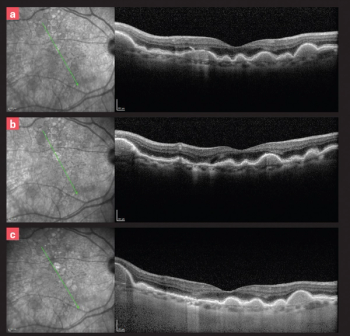
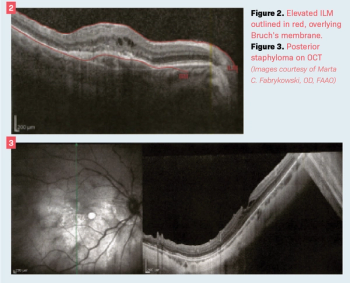
OCT showed inner retinal hyperreflectivity, diffuse retinal thickening with intraretinal fluid, and a serous macular detachment.
Evaluating accuracy of biometry is paramount in eyes with history of retinal pathology

As consistent use of digital devices remains a fact of life for people of virtually all ages, consider this expert advice

Faricimab is now the first and only FDA-approved drug to target two distinct pathways known to cause retinal disease that may lead to vision loss.

Timing is key in reducing the risk of developing vision loss or blindness.
Positive one-year results of Genentech’s phase III trials analyzing faricimab for treatment of wet AMD and DME were released today.

Expand your business by administering injectable treatments.

ASCENT, RegenXBio’s Phase III clinical trial conducted in partnership with AbbVie, is expected to enroll patients in the United States and Canada, with pivotal trials expected to support BLA submission for RGX-314 in 2024.
A review of monitoring guidelines, risk assessment, and treatment algorithms.