Retina
Latest News

Latest Videos

CME Content
More News

The 2 FDA registration trials for perfluorohexyloctane resulted in scientifically significant results for improvement of mild and moderate symptoms of dry eye disease.

Mary Beth Yackey, OD, and Michael Javaheri, MD, share their perspectives on the modernization of geographic atrophy identification and treatment.
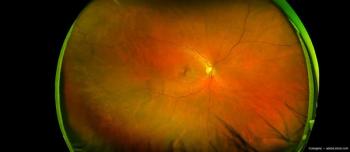
The AI-powered eye diagnostic innovator and medical devices and software solutions company have announced a collaboration to promote an AI diagnostic tool that assesses risk to brain health via retinal imaging.

The Diffusion Optics Technology, or DOT 0.2, works by utilizing thousands of light scattering elements to mimic natural contrast on the retina, which can slow myopia progression in child, according to the company.

The technology works by concentrating a specific band of light, typically 650 nm, into a laser beam to irradiate the retina.

The diagnostic screening technology is able to diagnose diabetic retinopathy by using 1 image taken of each eye with over 99% imageability.

The non-randomized, open label, safety and efficacy study will inject a sustained release implant in 5 participants.
Early detection of incipient geographic atrophy is critical to identify patients at greatest risk of progression and who may benefit most from newly approved complement inhibition therapies.

The AI-powered model works to detect early signs of glaucoma and makes iHealthScreen the first company to receive 2 US patents for AMD and glaucoma screening devices.

The goal of the grant funding from the Choroideremia Research Foundation is to support validation of functional vision assessments for patients with profound blindness.

The company stated that 46.2% of patients demonstrated a 1- or 2-step improvement in the Diabetic Retinopathy Severity Scale (DRSS) at 40 weeks in the Axpaxli arm, compared to 0% in the control arm.
The Phase 1/2 study includes a dose-escalation phase of the study featuring 3 cohorts each one receiving either a low, medium, or high dose of OCU410.

The Phase 3 study will have a sample size of 150 participants—one arm of 75 participants with the RHO gene mutation and the other arm with 75 participants that are gene agnostic.

The effort led to the creation of an artificial vitreous body for treating retinal detachment. This solution is based on a natural carbohydrate derived from algae.

MCO-010 demonstrated a statistically significant improvement of best-corrected visual acuity (BCVA) at week 52.

Rebecca Alexander, LCSW-R, MPH, has been gradually losing both her vision and hearing since she was a teenager due to Usher syndrome type 3. She discusses the importance of making practice accessible for those with vision loss in an exclusive interview.

Cleveland, Ohio’s Great Lakes Science Center Community Engagement Coordinator JonDarr Bradshaw details what those in the path of totality should expect, as well as the science center’s initiatives to keep eyes healthy during the event.
With The Ohio State University College of Optometry in the path of totality for this year's total solar eclipse, 1 optometry school has prepared its alumni, students, and doctors to share information with the public.

Prevent Blindness and the American Optometric Association have released resources and educational information to help the public and eye care providers prepare for the astronomical event on April 8.

Professor and chief of low vision rehabilitation service Patrick D. Yoshinaga, OD, MPH, FAAO, chats with Dr Korik about assistive tech, AI, and accessibility for patients with low vision.

The authors suggested genetic mutation may relax the body’s defenses and allow harmful bacteria to reach the eye.

The study focuses on similarities and differences in 11-cis-retinal and mutations in carotenoid cleavage enzymes.

Findings from Mass Eye and Ear’s collaborative study provides new insights regarding gene expression and post-transcriptional gene regulation.

The program, announced prior to ROP Awareness Week, has established an ROP-specific website with free resources for parents and eye care professionals.

According to the company, AGTC-501 was generally safe and well-tolerated and showed improvements in visual function at the 12-month analysis. The Phase 2/3 VISTA trial for AGTC-501 in XLRP expected to begin in in the first half of 2024.