
Retina
Latest News
Latest Videos

CME Content
More News

It’s time to act with a few rational and relatively modest proposals.

Comanagement is a key component for treatment of disease.

The new smart glasses utilize artificial intelligence to visually assist those with age-related macular degeneration and other retinal disorders causing vision loss.

Case complexity underscores the importance of interdisciplinary communication.

The study helped identify predisposing factors for severe SANS in astronauts.

A new analysis by researchers at the National Institutes of Health shows the benefit of taking AREDS2 formula in late AMD.

The 2-year Phase III data demonstrates Susvimo’s potential as an alternative to eye injections to treat diabetic macular edema and diabetic retinopathy.

Study investigators conducted a case-control study to investigate the corneal topography parameters and biomechanics in patients with alopecia areata.
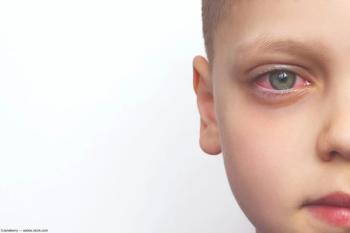
Pediatric TINU syndrome is rare, but responds well to treatment.
According to Genentech, faricimab-svoa is the first and only syringe that is prefilled with an FDA-approved bispecific antibody to treat wet AMD, DME and RVO.

The company also submitted a marketing authorization application with the European Medicines Agency.
Respecting the clinical importance of the pachychoroid spectrum is vital when analyzing OCT scans.

Rafieetary emphasizes the importance of follow-up plans and consistent support to prevent patients from dropping out of treatment, which can negatively impact their prognosis.

Neurotech's ocular implant NT-501 utilizes encapsulated cell therapy to slow the progression of macular telangiectasia type 2 (MacTel). The implant was given a PDUFA goal date of December 17, 2024.
Pathogenesis and detection of diabetic retinopathy.

The study evaluates the efficacy, safety, and tolerability of 2 dose levels of AGTC-501 for the treatment of X-linked retinitis pigmentosa.

Positive data from the GALE long-term extension study showed patients developed fewer new scotomatous points with 36 months of both continuous monthly and every-other-month treatment.

The French study found that a complete capsular removal seemed to be the safer option for pediatric patients with Marfan syndrome in terms of risk of retinal detachment.

The biotechnology company is developing the first protein-based artificial retina for patients with retinitis pigmentosa and age-related macular degeneration.

DF-003 is a first-in-class, oral, potent, highly selective alpha-kinase 1 (ALPK1), an inhibitor believed to be responsible for causing ROSAH syndrome.
The trial evaluates OCU410ST, a modifier gene therapy candidate utilizing an AAV delivery platform for the retinal delivery of the RORA gene. The gene therapy is being designed as a one-time treatment for life.

Deborah Ferrington, chief scientific officer at Doheny Eye Institute, details a session she gave on May 9 regarding mitochondria and how they change with health and in the diseased retina.

The 2 FDA registration trials for perfluorohexyloctane resulted in scientifically significant results for improvement of mild and moderate symptoms of dry eye disease.

Mary Beth Yackey, OD, and Michael Javaheri, MD, share their perspectives on the modernization of geographic atrophy identification and treatment.
The AI-powered eye diagnostic innovator and medical devices and software solutions company have announced a collaboration to promote an AI diagnostic tool that assesses risk to brain health via retinal imaging.

















































.png)


