John D. Gelles, OD, FAAO, FIAOMC, FCLSA, FSLS, FBCLA, shares highlights from his Vision Expo West presentation: "Corneal diagnostics: A-Z."

John D. Gelles, OD, FAAO, FIAOMC, FCLSA, FSLS, FBCLA, shares highlights from his Vision Expo West presentation: "Corneal diagnostics: A-Z."
John D. Gelles, OD, FAAO, FIAOMC, FCLSA, FSLS, FBCLA, discusses his presentation titled"Keratoconus 1, 2, 3: simple management," during Vision Expo West.

With the launch of dark adaptation, Heru is now the only platform on the AMD market to include contrast sensitivity and dark adaptation exams.

Azura has secured funding to support its 2-stage study evaluating the safety, tolerability, and efficacy of AZR-MD-001, its leading candidate, for patients with contact lens discomfort who show signs of meibomian gland dysfunction.

OCS-01 is a novel, high concentration, preservative free, topical formulation of dexamethasone that has the potential to be the first topical eye drop and non-invasive treatment for DME.
Ashley Tucker, OD, FAAO, FSLS, Dipl ABO, shares highlights from her discussion titled, "Myopia management: past, present, and future," presented during this year's 115th annual SCOPA meeting.
Ashley Tucker, OD, FAAO, FSLS, Dipl ABO, shares highlights from her presentation on amniotic membrane treatment for anterior segment disease during the South Carolina Optometric Physicians Association’s annual meeting.

Leo P. Semes, OD, FAAO, highlights his presentation on "Artificial intelligence and telemedicine," presented during the 115th annual SCOPA meeting.
Leo P. Semes, OD, FAAO, discusses his presentation on, "A new look at AMD," during the South Carolina Optometric Physicians Association's annual meeting.
Latest data from phase 3 studies finds increased effects over time with intravitreal pegcetacoplan for geographic atrophy secondary to age-related macular degeneration.
New DME treatments aim to extend time between intravitreal injections.

A recent clinical trial from the DRCR Retina Network examined a stepped regimen of anti-VEGF drugs bevacizumab and aflibercept.

IBI324 is a potential first-in-class ophthalmic recombinant human anti-VEGF-A and anti-Ang-2 bispecific antibody.

Proper screenings, treatment, management needed for this increasingly prevalent disease.
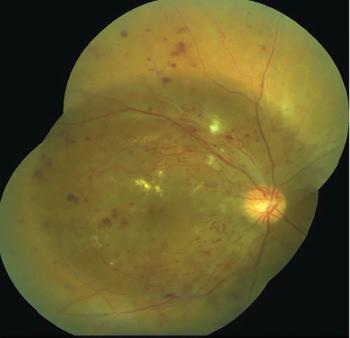
The patient’s ocular history was significant for severe nonproliferative diabetic retinopathy (NPDR) in the right eye (OD) and mild NPDR in the left eye (OS), which had been diagnosed 4 years previously at his last eye exam.

Korean investigators have found intermittent fasting may actually lead to a lower risk of developing AMD in the elderly population.

If approved, pegcetacoplan—an investigational, targeted C3 therapy—will be the first-ever treatment for GA.
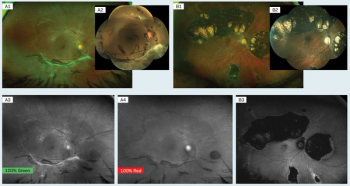
Advancements enable better diagnosis, treatment of patients.

Presented during the 2022 ASRS meeting, results find that over 60% of participants could be treated every 4 months at 2 years—an increase from 45% at year 1.
A decade after the conclusion of the NIH-funded AREDS2 study, researchers found that the AREDS2 formula also reduces the risk of lung cancer.

Trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.
A. Paul Chous, OD, discusses his presentation, “Current treatment of diabetes" during the AOA's Optometry Meeting.
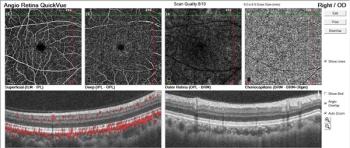
Case report details diagnosis based on imaging diagnostics.

Embracing this new model of care can allow optometric practices to flourish—and may enable earlier detection of conversion from intermediate dry to wet AMD.

Early diagnosis and intervention are crucial when managing this patient base.