Technology
Latest News
Latest Videos

CME Content
More News

Learn what's in the pipeline for eSight, as well as hear how their current technology helps patients.
Easy Anyama shares key takeaway points from "Vision care in the age of ChatGPT" and "Advances in AI and diagnosis for BIPOC populations" at Vision Expo West 2023 in Las Vegas.

This new platform incorporates the SILK procedure, allowing surgeons to perform refractive correction on patients with myopia.

How to tailor an eye examination and what technology to consider.
Managing patient expectations is key to selecting the best lens

Spectacle independence, nighttime glare symptoms often are key factors.
Investigators set out to evaluate how well an AI system works when integrated into a handheld smartphone-based retinal camera to screen patients for DR using 1 retinal image in each eye.

At NOA 2023, Brianna Rhue, OD, FAAO, spoke to us about Dr. Contact Lens, a practice management tool that allows patients to reorder lenses online, sends reminders for followup appointments, and more.

Take a look back at the first half of 2023 as we explore the role of AI in the eye care field in anticipation of the changes to come.
Marc Taub, OD, MS, FAAO, FCOVD, took a moment to speak to Optometry Times about exciting, dynamic technology that can improve experience in vision therapy rooms.
Carl Garbus, OD, FAAO, shares insights from his discussion that he copresented during the 2023 AOA Optometry's Meeting in Washington, DC.

Mile Brujic, OD, FAAO, shares an overview of his AOA 2023 presentation, which covered 12 new treatments all optometrists should know about.

Comanagement optimizes outcomes for patients who wear scleral lenses.
The project, a collaboration with researchers from the University of Maryland, holds promise for advancing new and more tolerable drug treatments for common chronic blinding eye diseases, including glaucoma and macular degeneration.

Artificial intelligence is the future of optometric disease monitoring.
This medical device is the first retinal eye-movement monitor for non-invasive, objective clinical assessments.

Mark Packer, MD, sat down with Sheryl Stevenson, Group Editorial Director, Ophthalmology Times®, to discuss his presentation on machine learning and predicting vision outcomes after cataract surgery at the ASCRS annual meeting in San Diego.
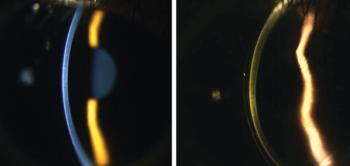
How to fit lenses with simple devices and when to expand your armature.

Neurofibromatosis type 1 (NF1) is a condition associated with significant systemic morbidity. Abnormal proliferation of cells derived from the neural crest can cause benign and malignant tumors throughout the body in NF1.

In addition to these tips on preventing digital eye strain, it is important to implement other eye-healthy habits.

A look back on what happened in optometry during the week of April 3-April 7.

Based on the initial ophthalmic findings, it is difficult but important to predict the patients’ progression to a specific level of disease severity.

Step up your standard of care, free up office space, and exceed patient expectations by taking advantage of these tools.

A look back on what happened in optometry during the week ofMarch 27-March 31.
Technologies that improve treatment of ONHD and similar pathologies.